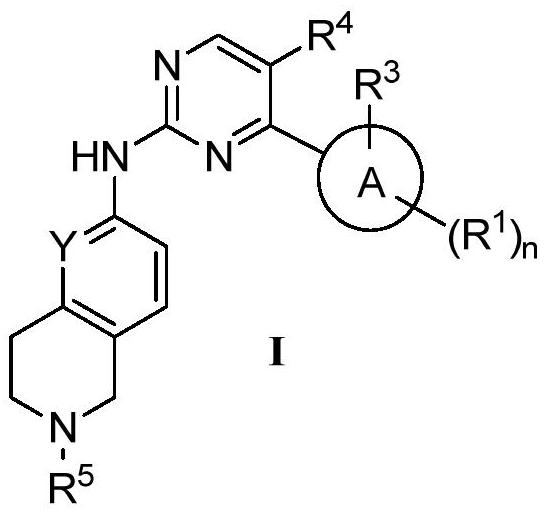
Nitrogen-containing condensed heterocyclic compound, its preparation method, intermediate, composition and application
A technology of fused heterocycles and compounds, applied in the field of nitrogen-containing fused heterocycles, can solve the problem of low inhibitory activity and achieve good inhibitory activity, good stability, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0254]
[0255] first step:
[0256] Compound 5-(2-aminopyrimidin-4-yl)-N-cyclopentyl-4-methylthiazol-2-amine (276 mg, 1 mmol) as shown in formula 1-1-a, 2-chloro-7 , 8-dihydro-1,6-naphthyridine-6(5H)-tert-butyl carboxylate (269mg, 1mmol), cesium carbonate (716mg, 2.2mmol), 4,5-bisdiphenylphosphine-9, 9-Dimethylxanthene (46mg, 0.08mmol), tris(dibenzylideneacetone) dipalladium (37mg, 0.04mmol) were added to 1,4-dioxane (8ml), under the protection of argon The temperature was raised to 110° C. for 12 hours. After cooling to room temperature, filter with diatomaceous earth, after the filtrate is concentrated, go through silica gel column chromatography (methanol / dichloromethane 0~10%) to obtain the compound 2-((4-(2- (Cyclopentylamino)-4-methylthiazol-5-yl)pyrimidin-2-yl)amino)-7,8-dihydro-1,6-naphthyridine-6(5hydrogen)-tert-butylcarboxy acid ester (280mg, 0.55mmol).
[0257] LC-MS: m / z: (M+H) + = 508.2.
[0258] Step two:
[0259] Compound 2-((4-(2-(cyclopentylamino)-4...
Embodiment 2
[0262]
[0263] first step:
[0264]Compound N-cyclopentyl-4-methyl-5-(2-((5,6,7,8-tetrahydro-1,6-naphthyridin-2-yl)amino as shown in formula 1-1 )pyrimidin-4-yl)thiazole-2-amine (140mg, 0.344mmol) and N-tert-butoxycarbonyl-(methylamino)acetaldehyde (130mg, 1.492mmol) and NaBH (OAc) 3 (220mg, 1.03mmol) was added into dichloromethane (10mL), and stirred at 45°C for 16 hours. The reaction solution was concentrated to obtain the compound (2-(2-((4-(2- (Cyclopentylamino)-4-methylthiazol-5-yl)pyrimidin-2-yl)amino)-7,8-dihydro-1,6-naphthyridin-6(5hydrogen)-yl)ethyl ) (Methyl) tert-butylcarboxylate (190 mg, 0.34 mmol) was used directly in the next step without further purification. LC-MS: m / z: (M+H) + = 565.3.
[0265] Step two:
[0266] Compound (2-(2-((4-(2-(cyclopentylamino)-4-methylthiazol-5-yl)pyrimidin-2-yl)amino)- 7,8-dihydro-1,6-naphthyridin-6(5hydro)-yl)ethyl)(methyl)tert-butylcarboxylate (190mg, 0.34mmol) was added to 2M HCl / EtOAc solution (2ml ), stirred at room ...
Embodiment 3
[0269]
[0270] Compound N-cyclopentyl-4-methyl-5-(2-((6-(2-(methylamino)ethyl)-5,6,7,8-tetrahydro as shown in formula 1-2 -1,6-naphthyridin-2-yl)amino)pyrimidin-4-yl)thiazole-2-ammonia (80mg, 0.17mmol) and aqueous formaldehyde (42mg, 0.51mmol) and NaBH (OAc) 3 (73 mg, 0.34 mmol) was added into methanol (5 mL), and stirred at room temperature for 16 hours. The reaction solution was concentrated and extracted with DCM / MeOH to obtain the compound N-cyclopentyl-5-(2-((6-(2-(dimethylamino)ethyl)-5,6,7,8-tetrahydro-1 , 6-naphthyridin-2-yl)amino)pyrimidin-4-yl)-4-methylthiazol-2-amine (80 mg, 0.167 mmol). 1 H NMR (400MHz, DMSO-d 6 )δ9.30(s,1H),8.36(d,J=5.4Hz,1H),8.21(d,J=6.9Hz,1H),8.03(d,J=8.4Hz,1H),7.42(d, J=8.5Hz, 1H), 6.95(d, J=5.5Hz, 1H), 3.99(dd, J=12.2, 5.8Hz, 1H), 3.57(s, 2H), 2.80(s, 4H), 2.59( t, J=6.9Hz, 2H), 2.49(s, 3H), 2.48-2.40(m, 2H), 2.18(s, 6H), 1.95(d, J=6.8Hz, 2H), 1.76-1.64(m ,2H),1.56(dt,J=10.3,6.3Hz,4H).
[0271] LC-MS: m / z: (M+H) + = 479.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com