Application of isoxuling in preparation of medicine for treating or preventing influenza virus infection
A technology of isoxalin and influenza virus, which is applied in the field of medicine, can solve the problem of not finding that isoxalin is anti-influenza virus, and achieve the effect of reducing clinical trial time, saving a lot of cost, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
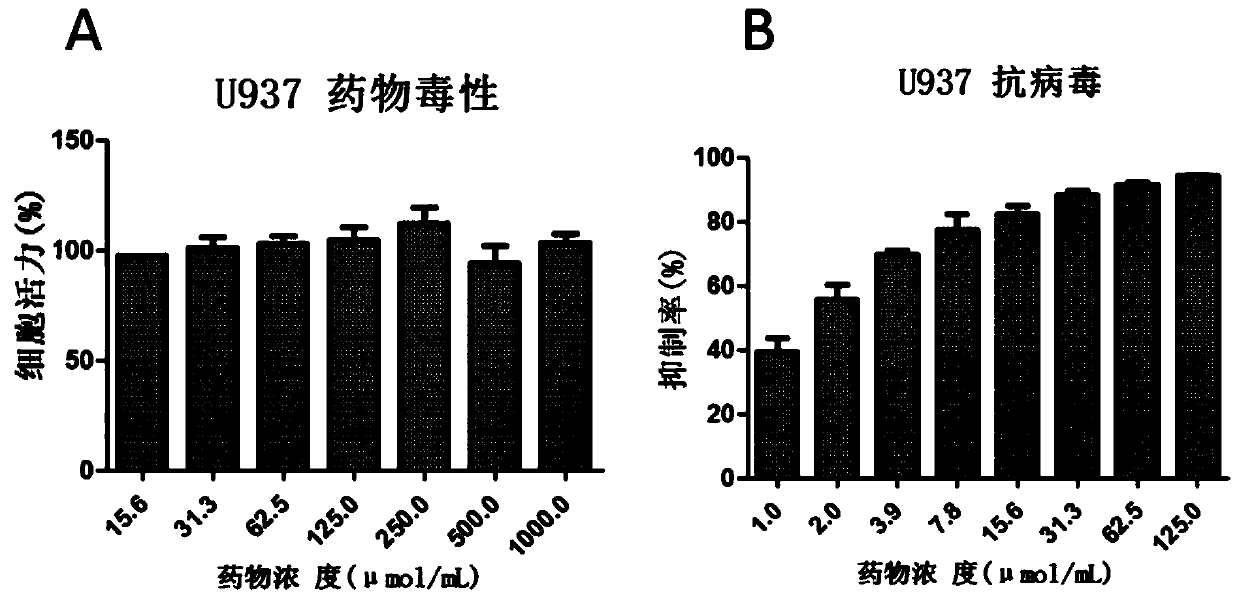
[0059] Example 1: Evaluation of the anti-influenza virus activity of Ikesuling in U937 cell line
[0060] 1 Cell culture
[0061] After two subcultures, the frozen and revived cells were expanded with RPMI-1640 medium containing 10% fetal bovine serum and double antibodies (penicillin 100U / ml, streptomycin 100ug / ml), and the inoculation density was not lower than 5x10 5 cell / ml, passage density not higher than 2x10 6 cell / ml.
[0062] 2 Cytotoxicity test of isoxuling
[0063] U937 cells by 1.5×10 5 Cells / well (volume 100 μl) were inoculated in a 96-well cell culture plate; the drug was prepared with 100 μl of culture medium (RPMI-1640 medium + 10% serum + double antibody) per well, and added to the corresponding cell wells for mixing. Seven concentration gradients were set for the drug, and two replicate wells were set for each gradient concentration. The final concentrations were 15.6 μM, 31.25 μM, 62.5 μM, 125.0 μM, 250.0 μM, 500.0 μM and 1000 μM. After culturing for 4...
Embodiment 2
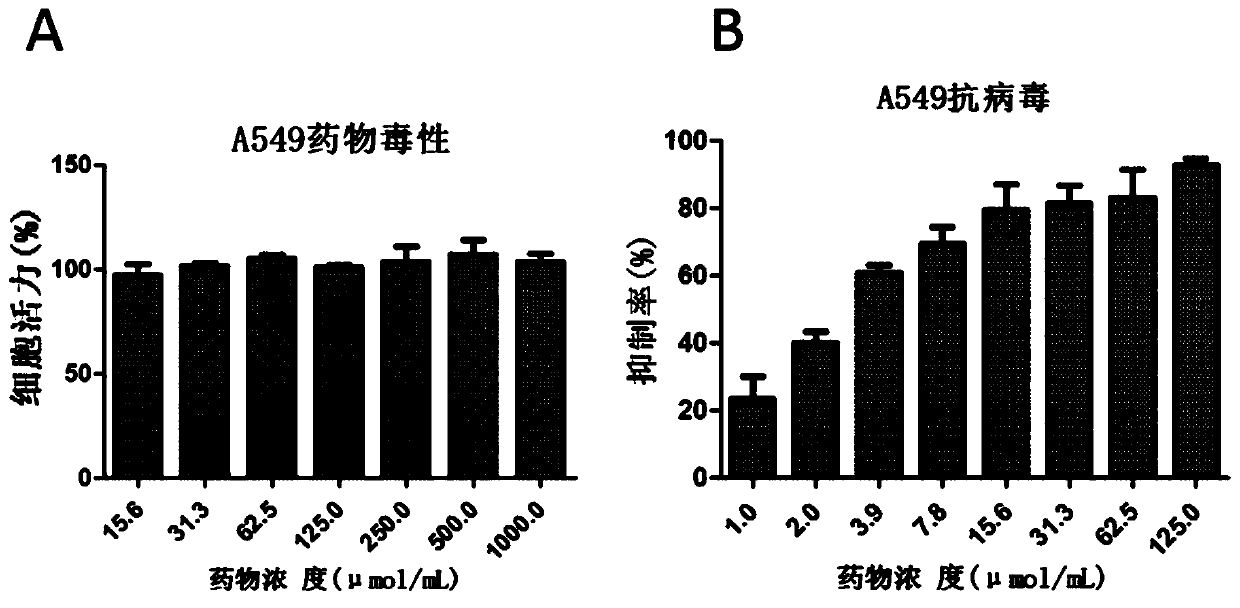
[0076] Embodiment 2: Evaluation of the anti-influenza virus activity of Ikesuling in A549 cell line
[0077] In this experiment, a method similar to that of Example 1 was used to evaluate the effect of Ixexulin in human lung epithelial cells A549.
[0078] Since A549 is an adherent cell, when detecting drug toxicity and antiviral activity, the number of cells required in a 96-well plate is 1.5×10 4 cells / well, and the cells need to be plated 24 hours in advance, and the medium should be changed before infection, and the rest of the operations are consistent with Example 1.
[0080] The result is as figure 2 As shown in middle A, after treatment of A549 cells with the highest concentration of 1000 μM for 48 hours, the viability of the cells was still not significantly different from that of the control group, indicating that the concentration of ioxalin had no toxicity to the cells, and its half-toxicity concentration CC 50 Greater than 1000 μM.
...
Embodiment 3
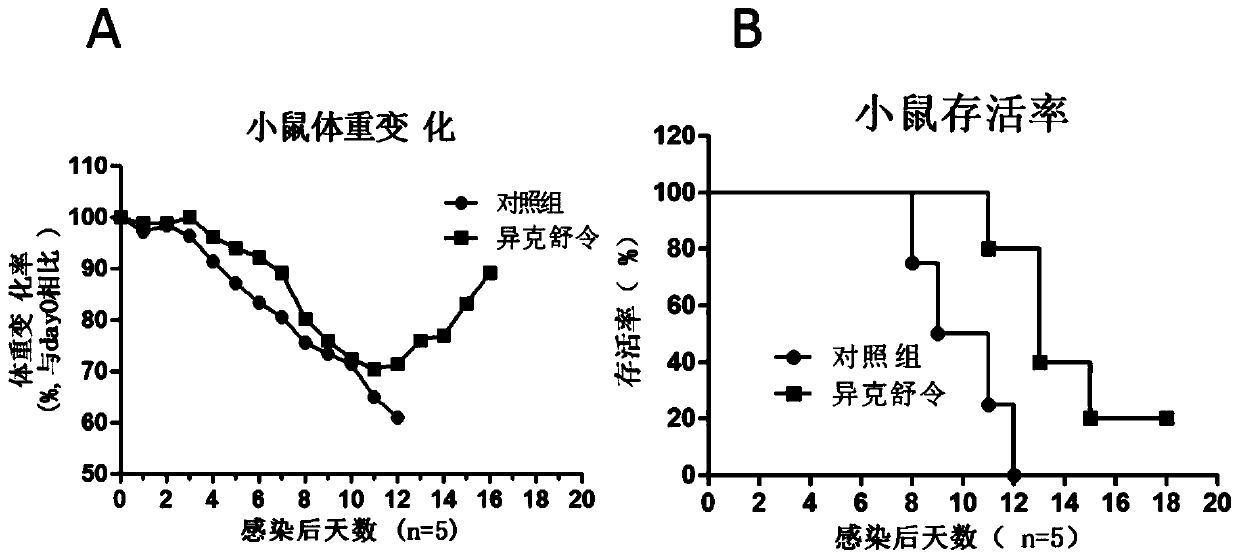
[0085] Example 3: Evaluation of the Anti-Influenza Virus Activity of Ikesuling in U937 Cell Lines
[0086] This embodiment uses H3N2 subtype (A / Human / Hubei / 3 / 2005) and H7N8 subtype (A / Duck / Hubei / 216 / 1983) and type B influenza virus (B / Human / Hubei / 1 / 2007) respectively The method of infecting U937 cell line was the same as that in Example 1. The results confirmed that Ixexulin significantly inhibited the replication of these three influenza viruses in a dose-dependent manner, and belonged to a broad-spectrum anti-influenza virus drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com