Mixed gene, standard plasmid and kit for detecting fusion gene as well as preparation method of standard plasmid
A technology of fusing genes and standard plasmids, applied in the field of molecular biology, can solve the problems of difficulty in guaranteeing the accuracy and consistency of detection results, small detection range, complicated operation, etc., to improve detection efficiency and accuracy, reduce detection costs, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] This example is the mixed gene, standard plasmid and preparation method used in the present invention.
[0058] The mixed gene for detecting fusion gene of the present invention, its gene sequence is as shown in SEQ ID NO: 1, and it is synthesized by gene by BCR-ABL fusion gene, AML-ETO fusion gene, PML-RARA fusion gene and ABL internal reference gene way to get.
[0059] Wherein, the BCR-ABL fusion gene is shown in SEQ ID NO: 2, the AML-ETO fusion gene is shown in SEQ ID NO: 3, the PML-RARA fusion gene is shown in SEQ ID NO: 4, and the ABL internal reference gene is shown in SEQ ID NO:5 shown.
[0060] Insert the above-mentioned mixed gene into a plasmid vector according to certain requirements to obtain a standard plasmid.
[0061] Wherein the plasmid vector is a PCU57 plasmid, the gene sequence of which is shown in SEQ ID NO:6.
[0062] The preparation method of the standard plasmid for detecting fusion gene of the present invention is as follows, comprising:
[...
Embodiment 2
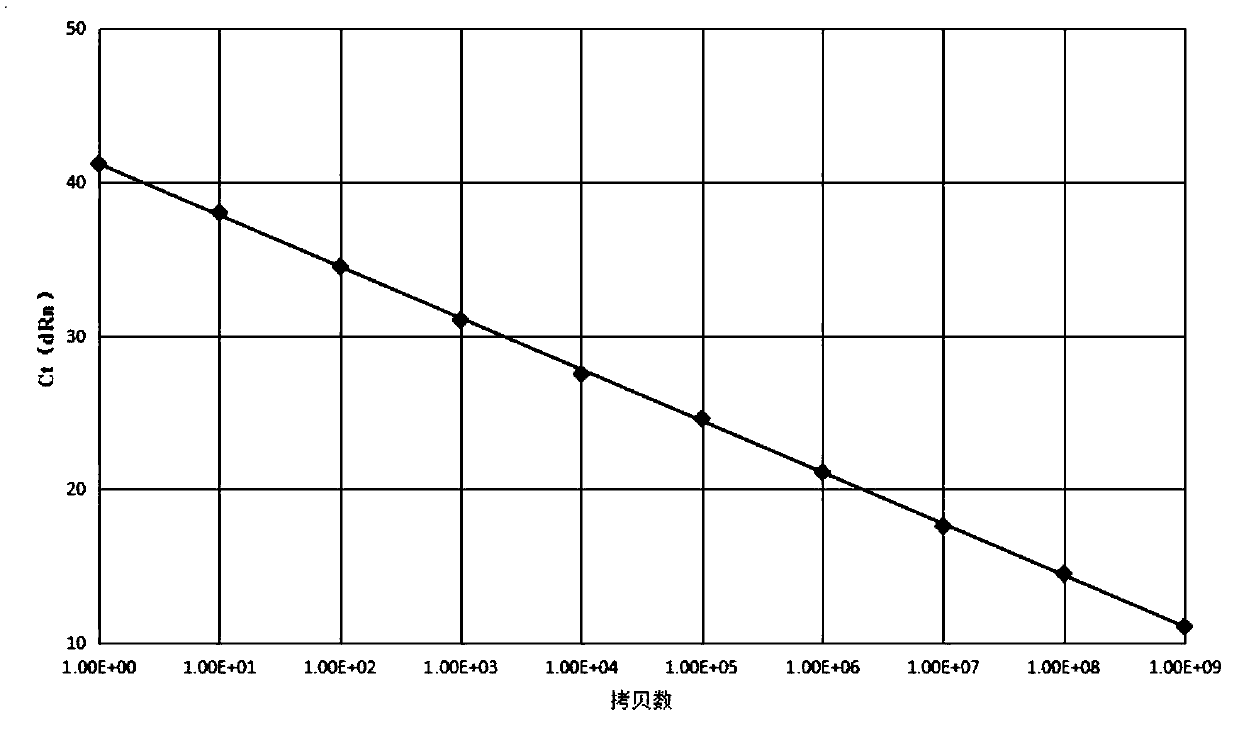
[0075] This example is to carry out the linearity verification and sensitivity verification of the ABL internal reference gene on the standard plasmid prepared in Example 1.
[0076] The specific steps of verification are as follows:
[0077] Step S51, measure the standard plasmid to obtain the concentration, absorbance and copy number of the standard plasmid.
[0078] The detection instrument is nanodrop2000, the absorbance is A260 absorbance, and the calculation formula of the copy number is shown in formula (1).
[0079] Copy number=50*A260 absorbance*6.02*10 14 / ((plasmid sequence length+mixed gene sequence length)*660)(1)
[0080] Step S52, performing gradient dilution of the standard plasmids to obtain standard plasmids with different copy numbers.
[0081] Among them, the copy numbers after serial dilution are 1*10 0 pcs / ml, 1*10 1 pcs / ml, 1*10 2 pcs / ml, 1*10 3 pcs / ml, 1*10 4 pcs / ml, 1*10 5 pcs / ml, 1*10 6 pcs / ml, 1*10 7 pcs / ml, 1*10 8 pcs / ml, 1*10 9 pieces / ...
Embodiment 3
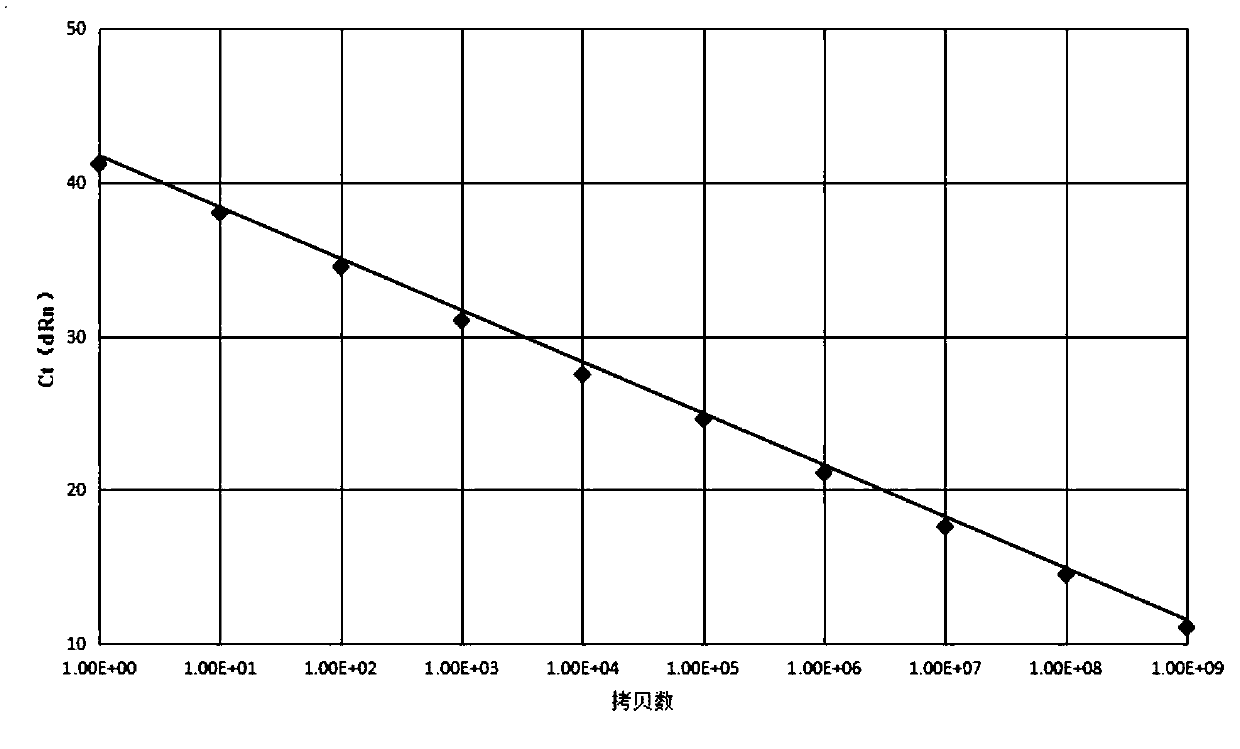
[0089] This example is to perform linearity verification and sensitivity verification of the BCR-ABL fusion gene on the standard plasmid prepared in Example 1.
[0090] The specific steps of verification are as follows:
[0091] Step S51, measure the standard plasmid to obtain the concentration, absorbance and copy number of the standard plasmid.
[0092] The detection instrument is nanodrop2000, the absorbance is A260 absorbance, and the calculation formula of the copy number is shown in formula (1).
[0093] Copy number=50*A260 absorbance*6.02*10 14 / ((plasmid sequence length+mixed gene sequence length)*660)(1)
[0094] Step S52, performing gradient dilution of the standard plasmids to obtain standard plasmids with different copy numbers.
[0095] Among them, the copy numbers after serial dilution are 1*10 0 pcs / ml, 1*10 1 pcs / ml, 1*10 2 pcs / ml, 1*10 3 pcs / ml, 1*10 4 pcs / ml, 1*10 5 pcs / ml, 1*10 6 pcs / ml, 1*10 7 pcs / ml, 1*10 8 pcs / ml, 1*10 9 pieces / ml.
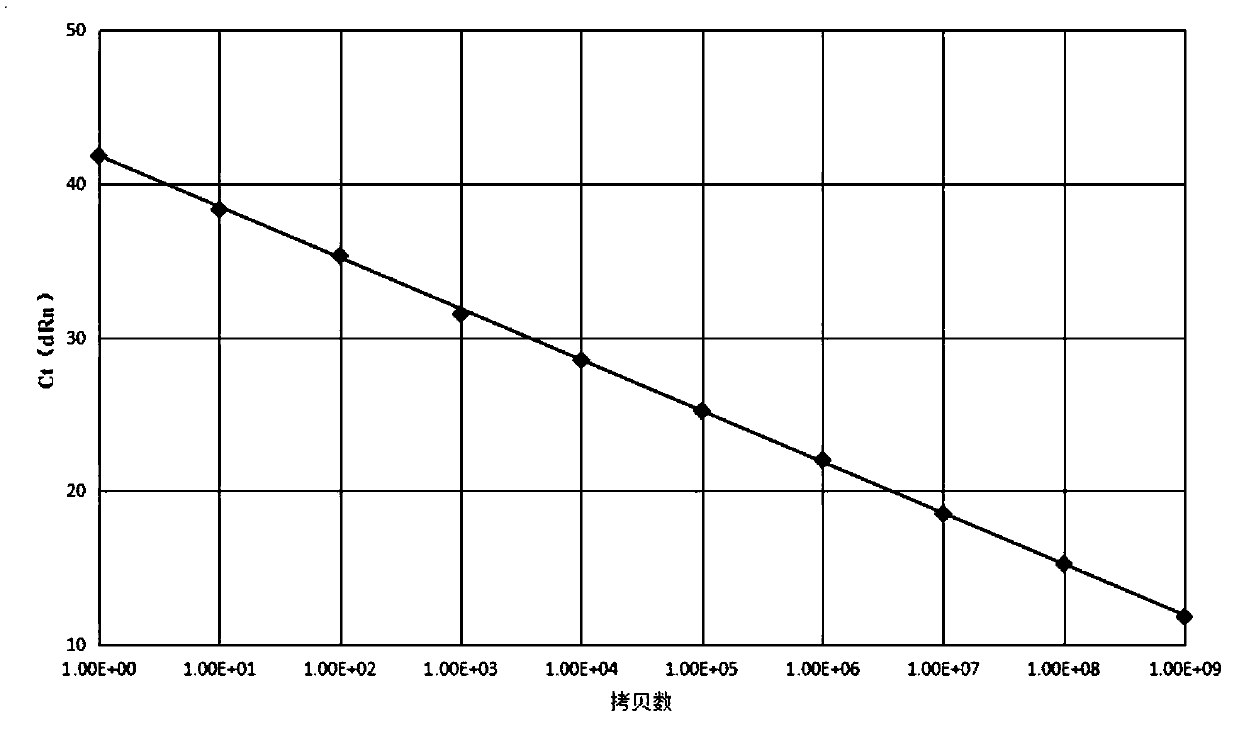
[0096] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com