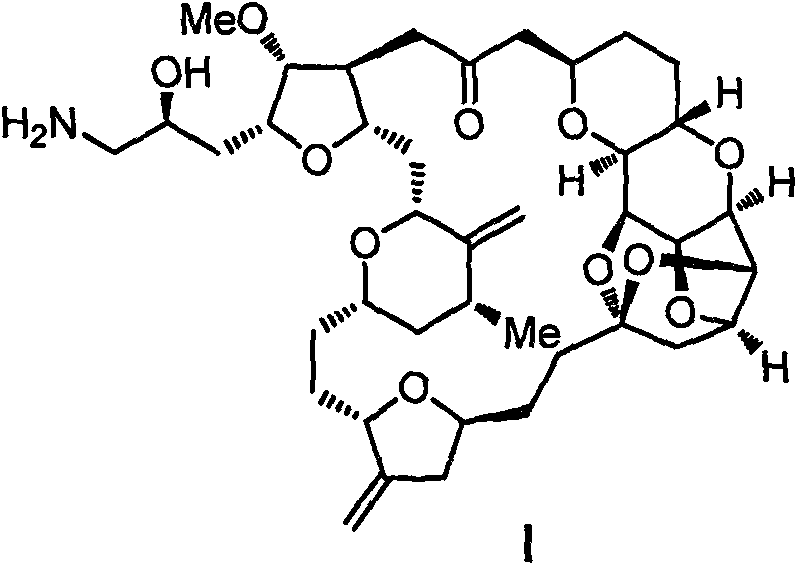
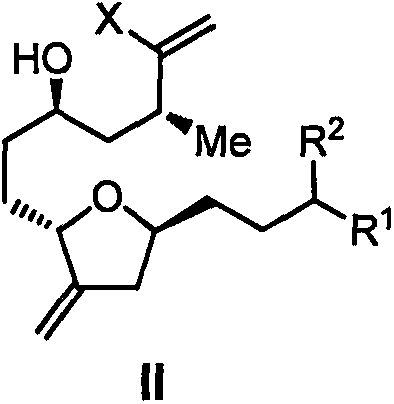
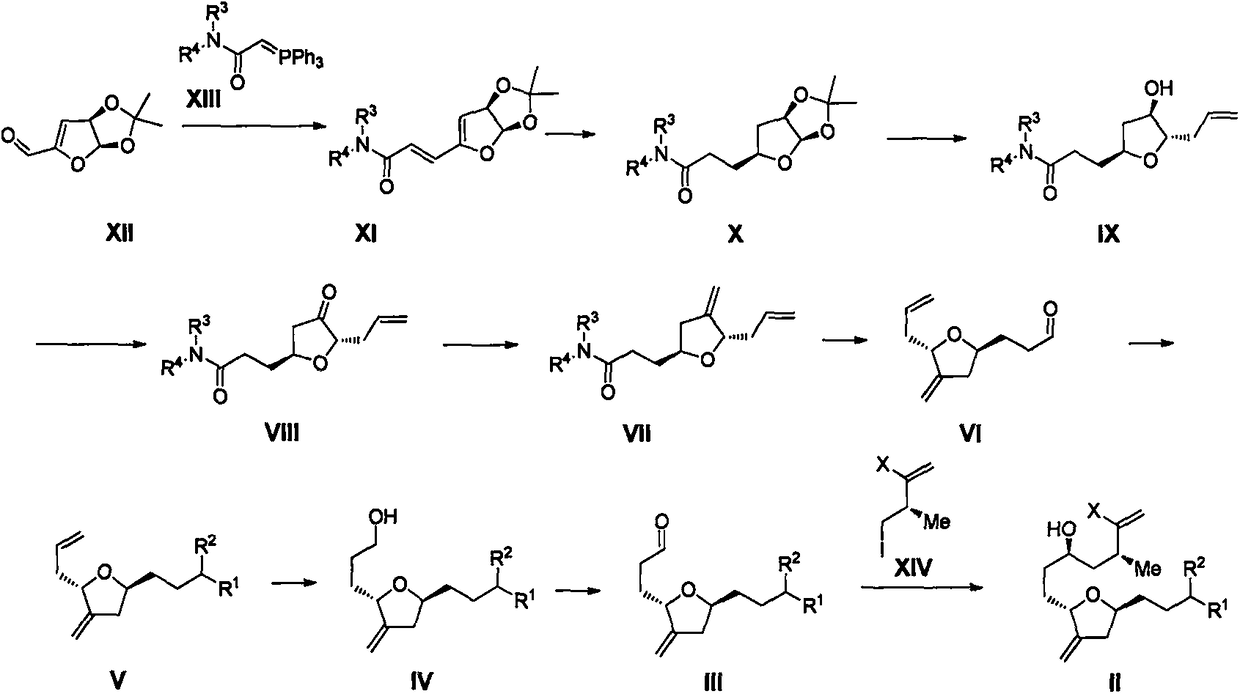
Eribulin intermediate and preparation method thereof
A compound and reaction technology, applied in the field of preparation of eribulin intermediates, can solve the problems of low diastereoselectivity, additional steps, high synthesis cost, etc., and achieve reduction of redox reactions, high diastereoselectivity, Synthetic green and efficient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Embodiment 1: Preparation of compound XIIIa
[0166] Dissolve N-methyl-N'-methoxy-chloroacetamide (132g, 959mmol) and triphenylphosphine (239g, 911mmol) in toluene, stir at room temperature for 15 hours, wash with 2N aqueous potassium hydroxide ( 1L*2), then washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 279g of XIIIa as a white powdery solid.
[0167] MS (ESI) m / z: 364 (M+H + )
[0168] 1 H NMR (400MHz, Chloroform-d) δ7.82-7.61(m, 6H), 7.63-7.52(m, 3H), 7.50-7.43(m, 6H), 3.75(s, 3H), 3.56(s, 1H ), 3.10(s, 3H).
Embodiment 2
[0169] Example 2: Preparation of Compound XIa
[0170] Compound XII (19.4g, 114mmol) was dissolved in 100mL of acetonitrile, and compound XIIIa (46g, 114mmol) was added in three batches. The reaction solution was stirred at room temperature for 10min, and the reaction solution was concentrated and separated by column chromatography to obtain 31g of XIa. Yellow viscous oil.
[0171] MS (ESI) m / z: 256 (M+H + )
[0172] 1 H NMR (400MHz, Chloroform-d) δ7.15(d, J=15.4Hz, 1H), 6.91(d, J=15.4Hz, 1H), 6.17(d, J=5.3Hz, 1H), 5.54(d , J=2.6Hz, 1H), 5.39(dd, J=5.3, 2.6Hz, 1H), 3.76(s, 3H), 3.30(s, 3H), 1.48(s, 3H), 1.45(s, 3H) .
Embodiment 3
[0173] Embodiment 3: preparation compound Xa
[0174] Compound XIa (31g, 114mmol) was dissolved in 500mL of tetrahydrofuran, added with 10% Pd / C (water content 60%), hydrogenated at 60°C for 19h under normal pressure, the reaction was complete, filtered and concentrated to obtain 24.4g of colorless Xa Oil.
[0175] MS (ESI) m / z: 260 (M+H + )
[0176] 1 H NMR (400MHz, Chloroform-d) δ 5.76 (d, J = 4.0Hz, 1H), 4.72 (ddd, J = 6.3, 4.0, 1.3Hz, 1H), 4.30-4.03 (m, 1H), 3.68 ( s, 3H), 3.16(s, 3H), 2.67-2.53(m, 2H), 2.32-2.04(m, 2H), 1.92(m, 2H), 1.54(s, 3H), 1.30(s, 3H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com