A kind of preparation method of unsymmetrical azobenzene
An azobenzene and asymmetric technology, applied in the field of preparation of asymmetric azobenzene, can solve the problems of low yield of asymmetric azobenzene, limited types of substituent groups, complicated preparation process, etc. Commercialized, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention provides a preparation method of asymmetric azobenzene, which comprises dissolving 6-chloro-2-carbonylbicyclo[3.1.0]hexane-6-carboxylate compounds in an organic solvent, adding aromatic hydrazine inorganic acid in sequence Salts and alkaline substances cause a ring-opening reaction to obtain unsymmetrical azobenzene after separation and purification. The structural formula of the 6-chloro-2-carbonylbicyclo[3.1.0]hexane-6-carboxylate compound is shown in formula (1):
[0029]
[0030] In formula (1), R 1 and R 2 each independently selected from a hydrogen atom, a halogen atom, an alkyl group, an aryl group or a substituted aryl group; wherein the aryl group is selected from benzene, naphthalene, biphenyl, anthracene, phenanthrene, furan, thiophene, indole, thiazole and benzene And thiazole, the substituted aromatic group has 1 to 5 substituting groups, and the substituting groups are selected from halogen atoms, hydroxyl groups, mercapto groups, cyano ...
Embodiment 1
[0046] Step (1): Dissolve 1642mg (20mmol) of 2-cyclopentenone and 2859mg (20mmol) of methyl dichloroacetate in N,N-dimethylformamide (100ml), and add 7820mg of cesium carbonate in batches under stirring (24mmol), react at room temperature for 48 hours. After the reaction, extracted with dichloromethane, washed with saturated brine to remove N,N-dimethylformamide, combined organic phases, dried over anhydrous sodium sulfate, filtered, concentrated by rotary evaporation under reduced pressure, and then separated by silica gel column method to obtain 2.94 g of methyl 6-chloro-2-carbonylbicyclo[3.1.0]hexane-6-carboxylate, the yield was 78%.
[0047]
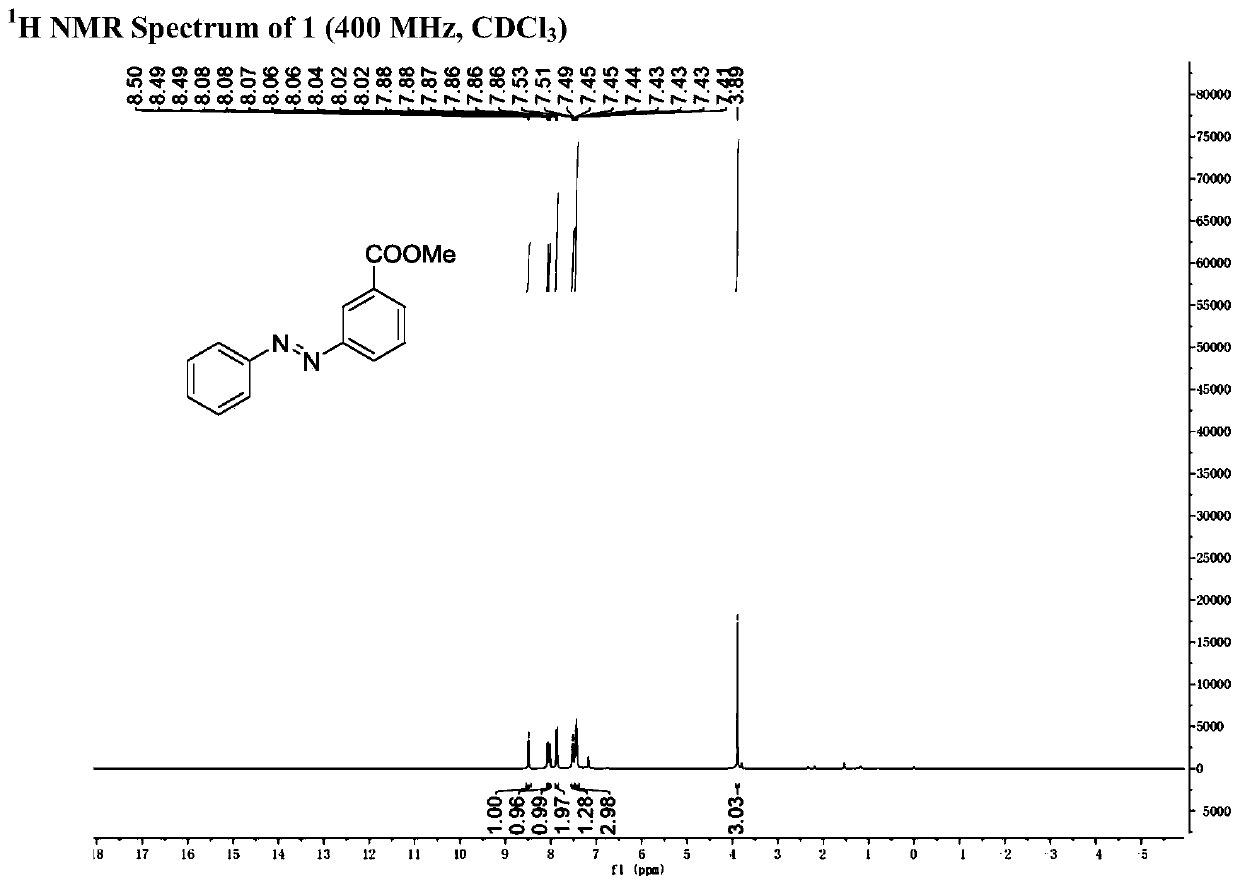
[0048] 1 H NMR (400MHz, CDCl 3 )δ(ppm)3.73(s,3H),2.73(t,J=6.4Hz,1H),2.64(d,J=6.4Hz,1H),2.48–2.36(m,1H),2.27–2.11(m ,3H).
[0049] Step (2): 188mg (1mmol) of methyl 6-chloro-2-carbonylbicyclo[3.1.0]hexane-6-carboxylate obtained in step (1) was dissolved in 5ml of toluene in a reaction test tube equipped with magnetic stirring ...
Embodiment 2
[0053] Step (1) is identical with embodiment 1
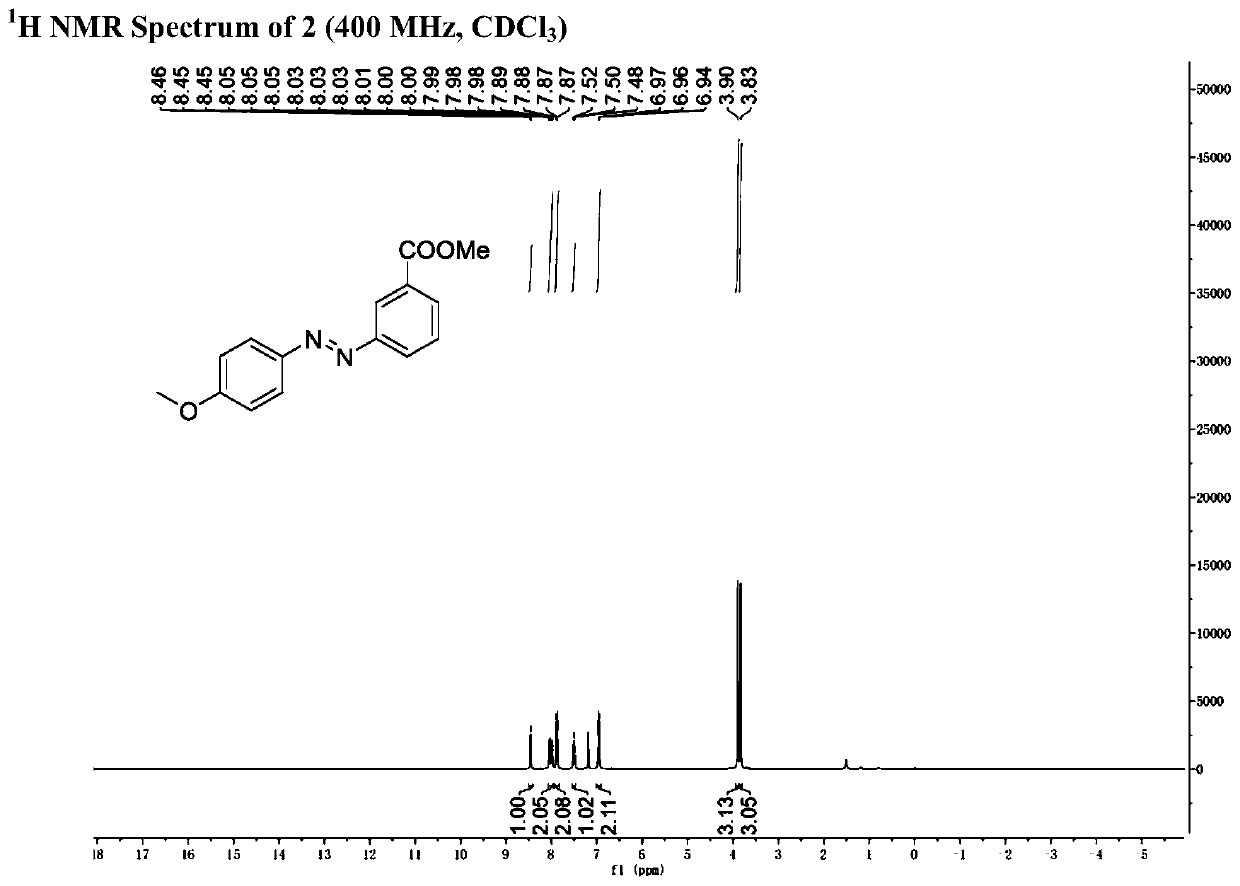
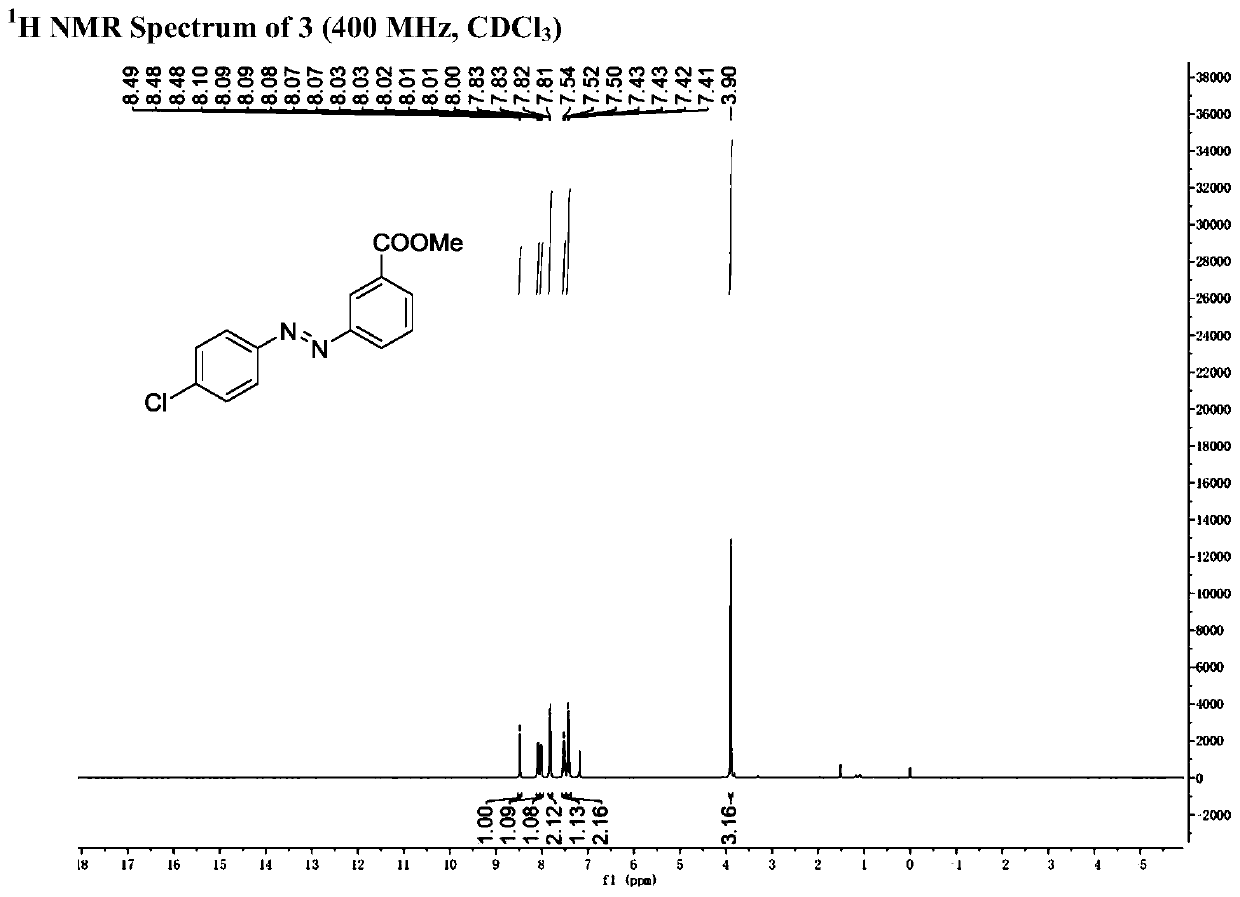
[0054] Step (2): 188mg (1mmol) of 6-chloro-2-carbonylbicyclo[3.1.0]hexane-6-methyl carboxylate obtained in step (1) was dissolved in 5ml N , to N-dimethylformamide, add 174mg phenylhydrazine hydrochloride (1.2mmol) and 166mg potassium carbonate (1.2mmol) successively; the mixture was stirred and reacted at 120°C for 24 hours, after the reaction, saturated saline Washing, extraction with dichloromethane, combined organic phases, drying over anhydrous sodium sulfate, filtration, concentration under reduced pressure, and separation by silica gel column chromatography to obtain trans-3-formyl azobenzene 125 mg, yield 52 %.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com