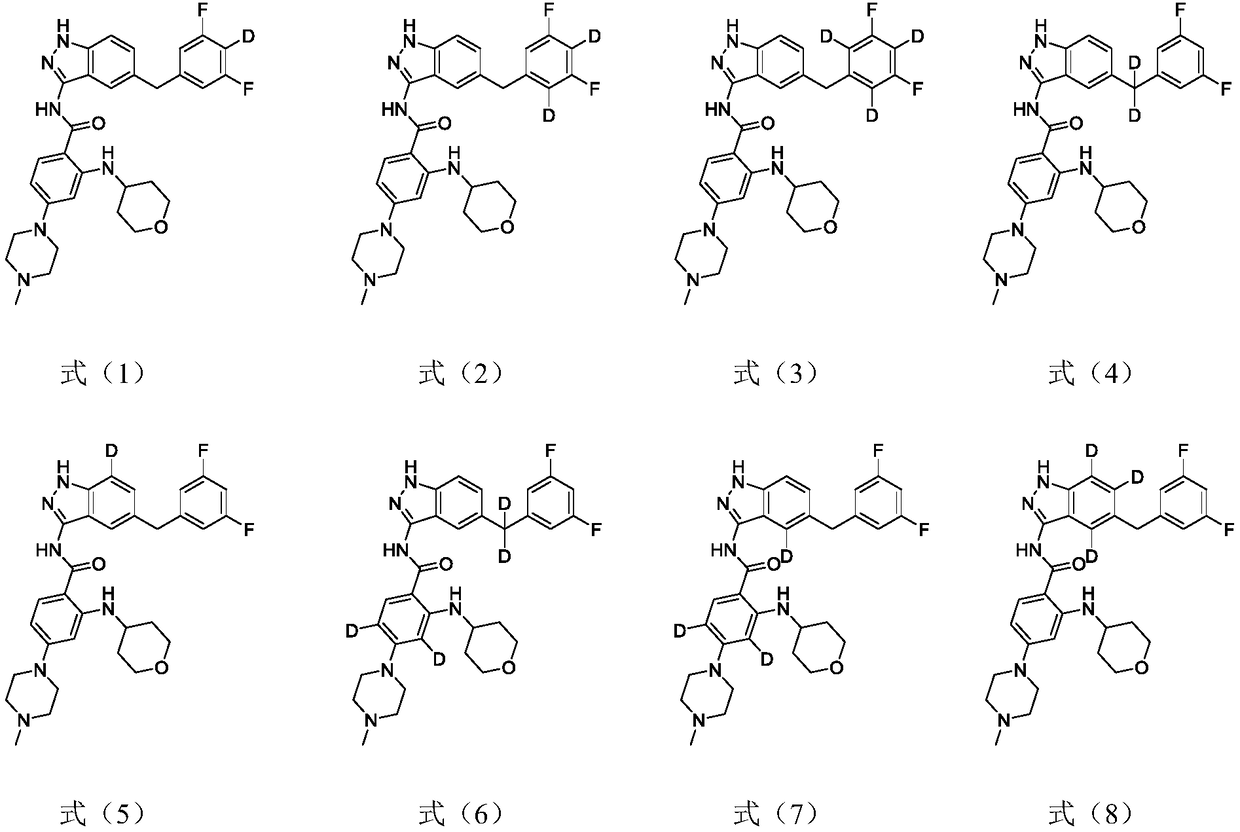
Indazole compound for inhibiting kinase activity as well as composition and application thereof
A compound, indazole technology, applied in the field of substituted indazole compounds, can solve problems such as poor absorption, distribution, metabolism and/or excretion, cost of side effects treatment, unmet clinical needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemistry of protecting groups can be found in, eg, Wuts and Greene, Protective Groups in Organic Synthesis, 4th Edition, John Wiley & Sons: New Jersey, (2006), which is incorporated herein by reference in its entirety.
[0102] The reaction can be monitored according to any suitable method known in the art. For example, spectroscopic means such as nuclear magnetic resonance (NMR) spectroscopy (e.g. 1 H or 13 C), infrared (IR) spectroscopy, spectrophotometry (e.g., UV-visible), mass spectroscopy (MS)) or by chromatographic methods such as high performance liquid chromatography (HPLC) or thin layer chromatography (TLC) Product formation was monitored.
[0103] Pharmaceutical compositions, prep...
Embodiment 1
[0135] Example 1 Preparation of N-(5-(3,5-difluorobenzyl)-1H-indazol-3-yl)-4-(4-(methyl-d 3 ) piperazin-1-yl)-2-((tetrahydro-2H-pyran-4-yl) amino) benzamide, namely compound T-1, molecular formula is as follows:
[0136]
[0137] Synthesize using the following route:
[0138]
[0139]
[0140] Synthesis of Step 1 Compound 3
[0141] Add toluene (100mL), compound 1 (5g, 30.30mmol), compound 2 (6.9g, 33.33mmol) and K 3 PO 4 (12.8g, 60.60mmol), vacuumize and replace with nitrogen, add Pd(PPh 3 ) 4 (770mg, 0.67mmol), vacuumize again and replace with nitrogen three times, heat up to 110°C, keep stirring for 5h. Cool to room temperature, add ethyl acetate (200 mL) to form a solid and filter, the filtrate is concentrated, and the residue is passed through a silica gel column to obtain 5.4 g of a white solid, yield 72.2%. 1 H NMR (400MHz, CDCl 3 )δ7.43-7.39(m,2H),7.18(t,J=8.8Hz,1H),6.73-6.66(m,3H),3.96(s,2H).
[0142] Synthesis of Step 2 Compound 4
[0143]Add n-buta...
Embodiment 2
[0162] Example 2 Preparation of N-(5-(3,5-difluorobenzyl)-1H-indazol-3-yl)-4-(4-methylpiperazin-1-yl-2,2,3, 3,5,5,6,6-d 8 )-2-((tetrahydro-2H-pyran-4-yl)amino)benzamide, i.e. compound T-2, molecular formula is as follows:
[0163]
[0164] Adopt the following synthetic route:
[0165]
[0166]
[0167] Step 1 Synthesis of Compound 18.
[0168] Compound 17 (928 mg, 10.8 mmol) was added to a solution of compound 6 (2 g, 8.3 mmol) in DMF (10 mL) under magnetic stirring, and the mixture was stirred at room temperature overnight. Add 50 mL of water, stir for 20 minutes, a large amount of solid precipitates, filter, wash with water (20 mL), and dry to obtain 1.9 g of yellow solid, yield 74.5%. LC-MS(APCI):m / z=213.1(M+1-100) + ,313.1(M+1) + .
[0169] Synthesis of step 2 compound 19
[0170]To a solution of compound 18 (1.9 g, 6.19 mmol) in acetonitrile (10 mL) under magnetic stirring, TsOMe (1.75 g, 9.28 mmol) and K 2 CO 3 (2.56g, 18.6mmol), the mixture was stirred...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com