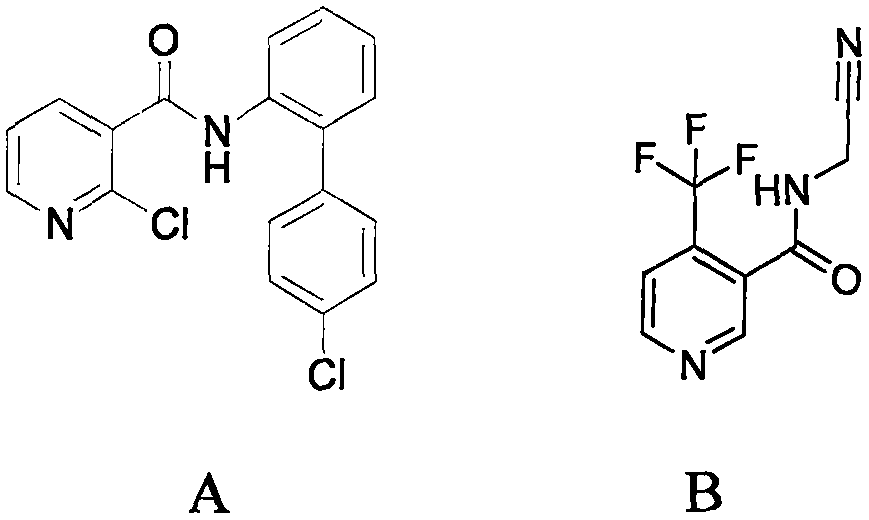
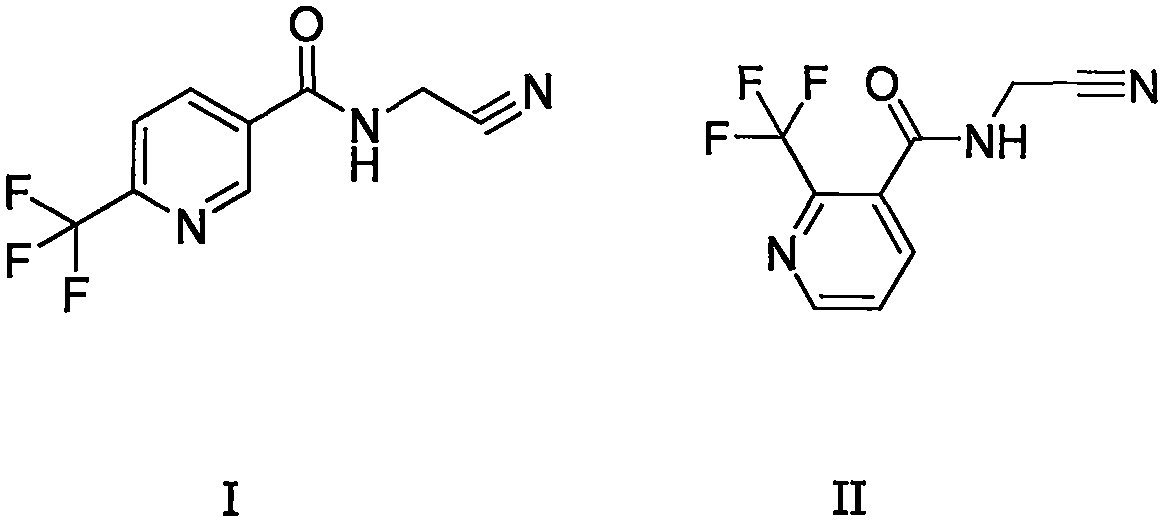
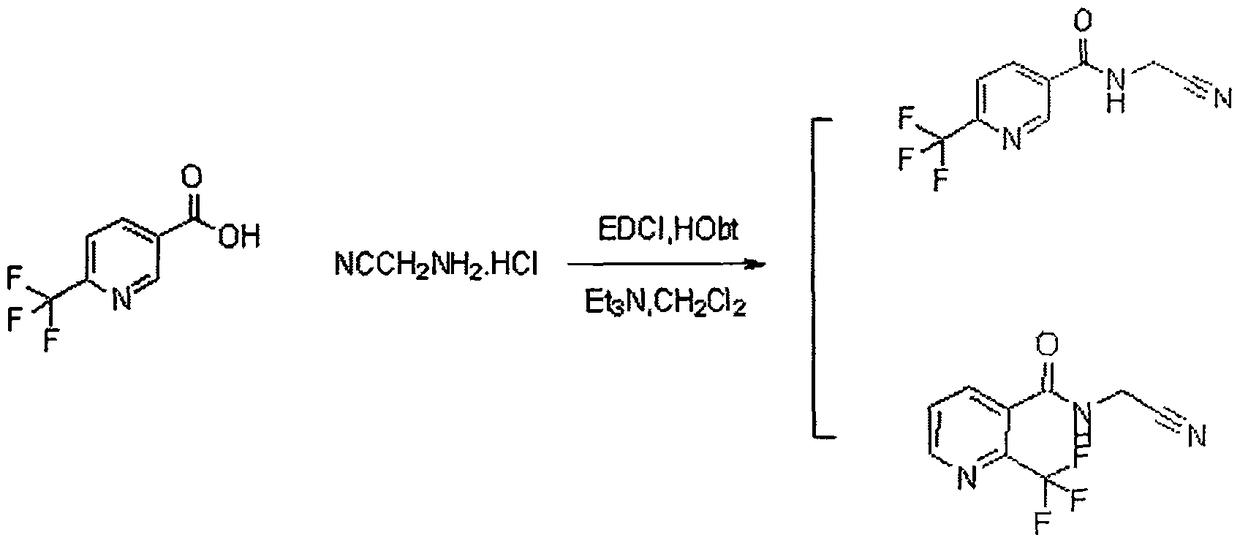
Trifluoromethylpicolinamide-containing compound and preparation method and application thereof
A technology of trifluoromethyl pyridine amide and trifluoromethyl pyridine, which is applied in the field of organic chemical synthesis, can solve the problems of poor environmental compatibility, and the herbicide is not easily degraded, and achieves low toxicity, convenient operation and good environmental compatibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] In one embodiment, the preparation method at least includes the following steps:
[0023] Using 2-trifluoromethylpyridine-3-carboxylic acid and aminoacetonitrile hydrochloride to carry out condensation acylation reaction under the conditions of organic solvent, alkali and condensing agent to obtain trifluoromethylpyridine amides compounds.
[0024] The above-mentioned preparation method is further explained below:
[0025] Preferably, the molar ratio of the 2-trifluoromethylpyridine-3-carboxylic acid to aminoacetonitrile hydrochloride is 1:1.0-1.2, so as to ensure the sufficient progress of the condensation reaction.
[0026] Preferably, the organic solvent is dichloromethane or chloroform.
[0027] Preferably, the condensing agent is at least one of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 1-hydroxybenzotriazole (HOBt) .
[0028] Preferably, the base is triethylamine or N,N-diisopropylethylamine, which provides a basic environment for th...
Embodiment 1
[0034] Preparation of structural formula I containing trifluoromethylpyridine amides
[0035] 2-trifluoromethylpyridine-5-carboxylic acid (0.191g, 1.0mmol) and aminoacetonitrile hydrochloride (0.081g, 1.0mmol) were dissolved in 25mL of dichloromethane, triethylamine (0.202g, 2.0mmol) was added ), then added EDC (0.287mg, 1.5mmol), HOBt (0.20g, 1.5mmol), and reacted at 25°C for 3h. Washing (20ml*1), drying with anhydrous sodium sulfate, precipitation to obtain a crude product, the crude product was purified by column chromatography to obtain a white solid, m.p.83-85°C, yield: 88%. 1H NMR (300MHz, DMSO) δ: 9.76-9.53 (m, 1H, pyridine), 9.16 (s, 1H, pyridine), 8.48 (d, J=9.2Hz, 1H, pyridine), 8.08 (d, J=9.2 Hz, 1H, pyridine), 4.39 (d, J=7.6Hz, 2H, CH2).
Embodiment 2
[0037] Preparation of structural formula II containing trifluoromethylpyridine amides
[0038] 2-trifluoromethylpyridine-3-carboxylic acid (0.191g, 1.0mmol) and aminoacetonitrile hydrochloride (0.081g, 1.0mmol) were dissolved in 25mL of dichloromethane, triethylamine (0.202g, 2.0mmol) was added ), then added EDCI (0.287mg, 1.5mmol), HOBt (0.20g, 1.5mmol), reacted at 25°C for 2.5h, TLC detected that the reaction was complete, the reaction solution was washed with water (20ml*2), washed with saturated brine (20ml *1), dried over anhydrous sodium sulfate, and precipitated to obtain a crude product, which was obtained by column chromatography as an off-white solid, m.p.90-93°C, yield: 79.5%. 1H NMR (300MHz, DMSO) δ9.43(s, 1H, NH), 8.83(d, J=4.3Hz, 1H, pyridine), 8.07(d, J=7.6Hz, 1H, pyridine), 7.90-7.70( m, 1H, pyridine), 4.37 (d, J=7.2Hz, 2H, CH2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com