PH response type star-like amphiphilic polymer and preparation method thereof
An amphiphilic polymer and star-shaped technology, which is applied in the field of pH-responsive star-shaped amphiphilic polymers and their preparation, can solve the problems of less structure types, and achieves low molecular weight distribution coefficient, good application prospect and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
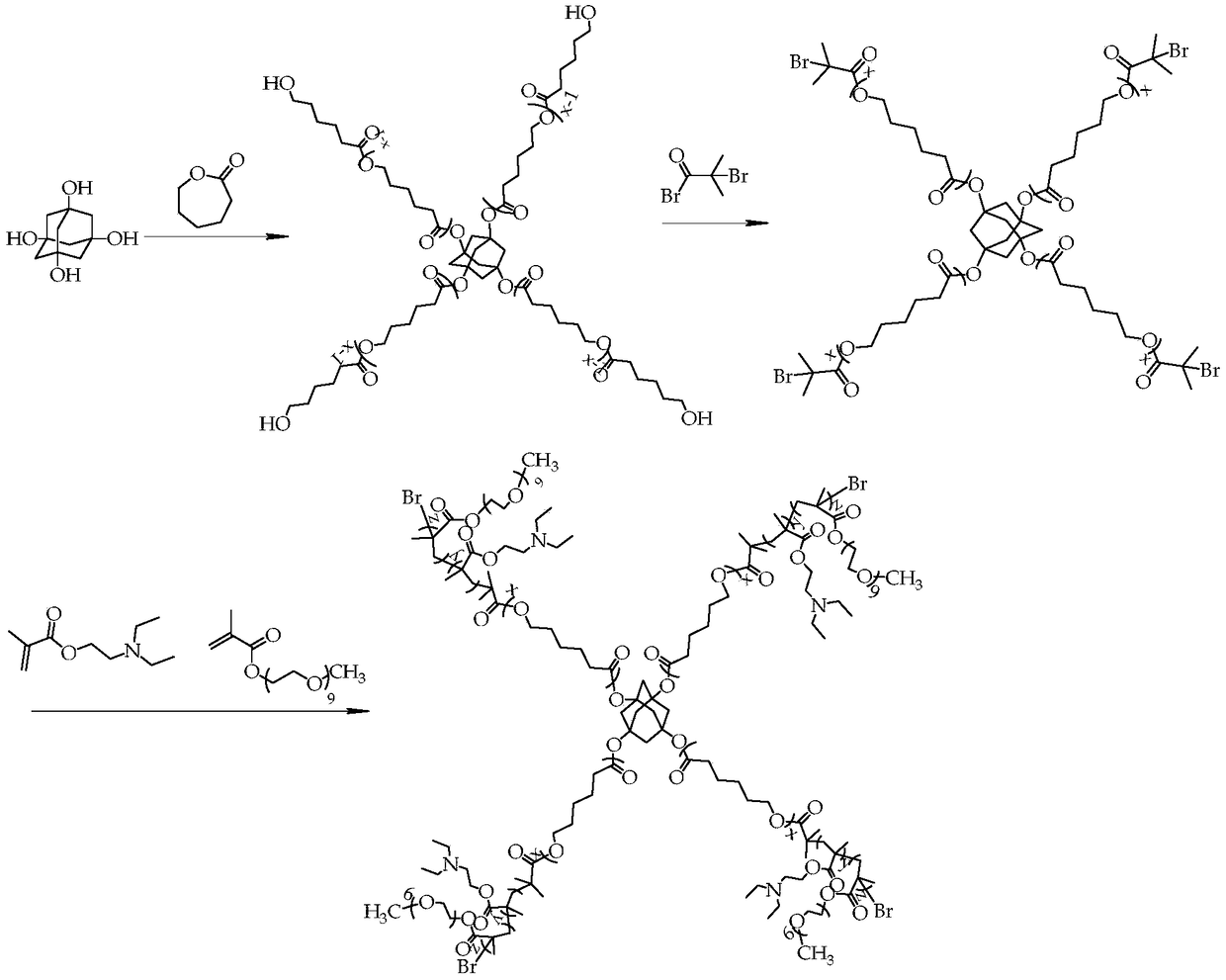
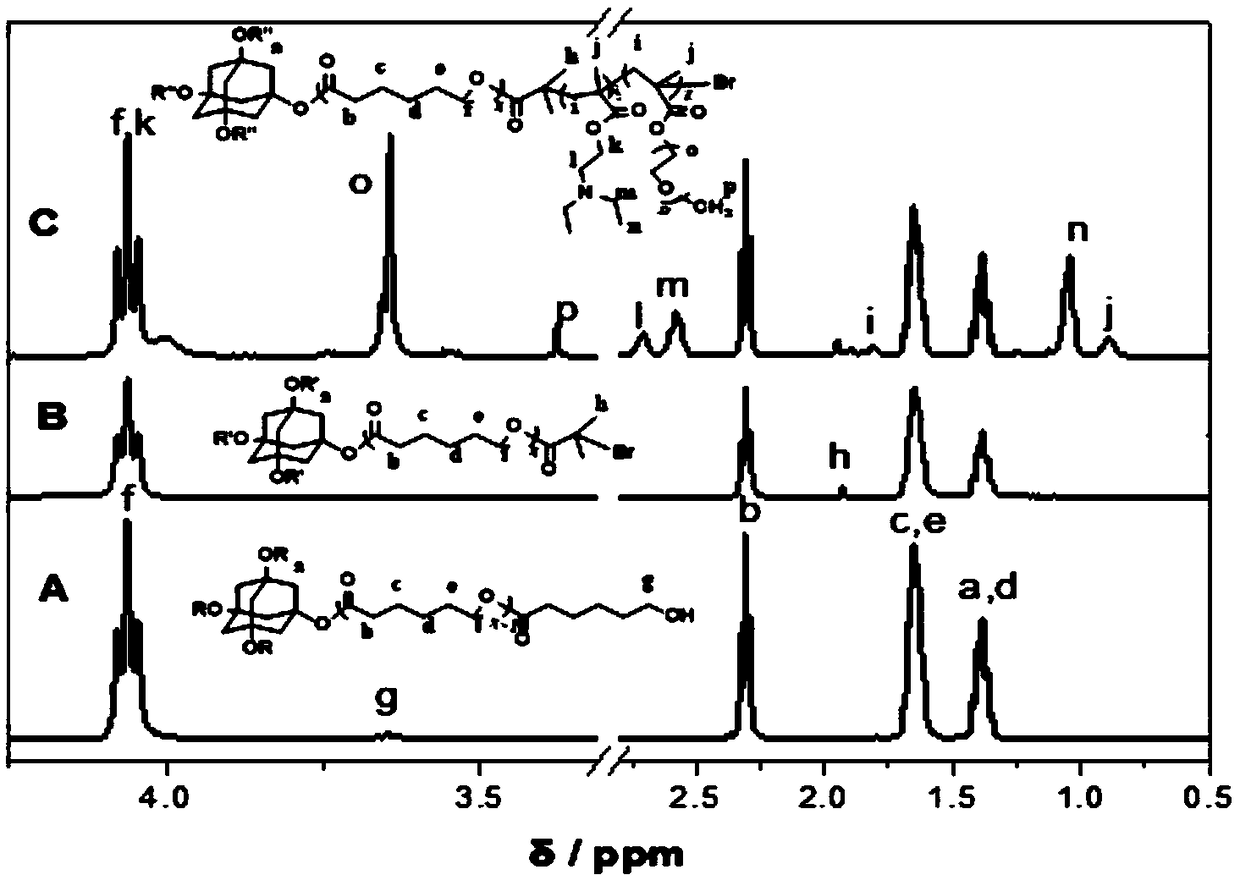
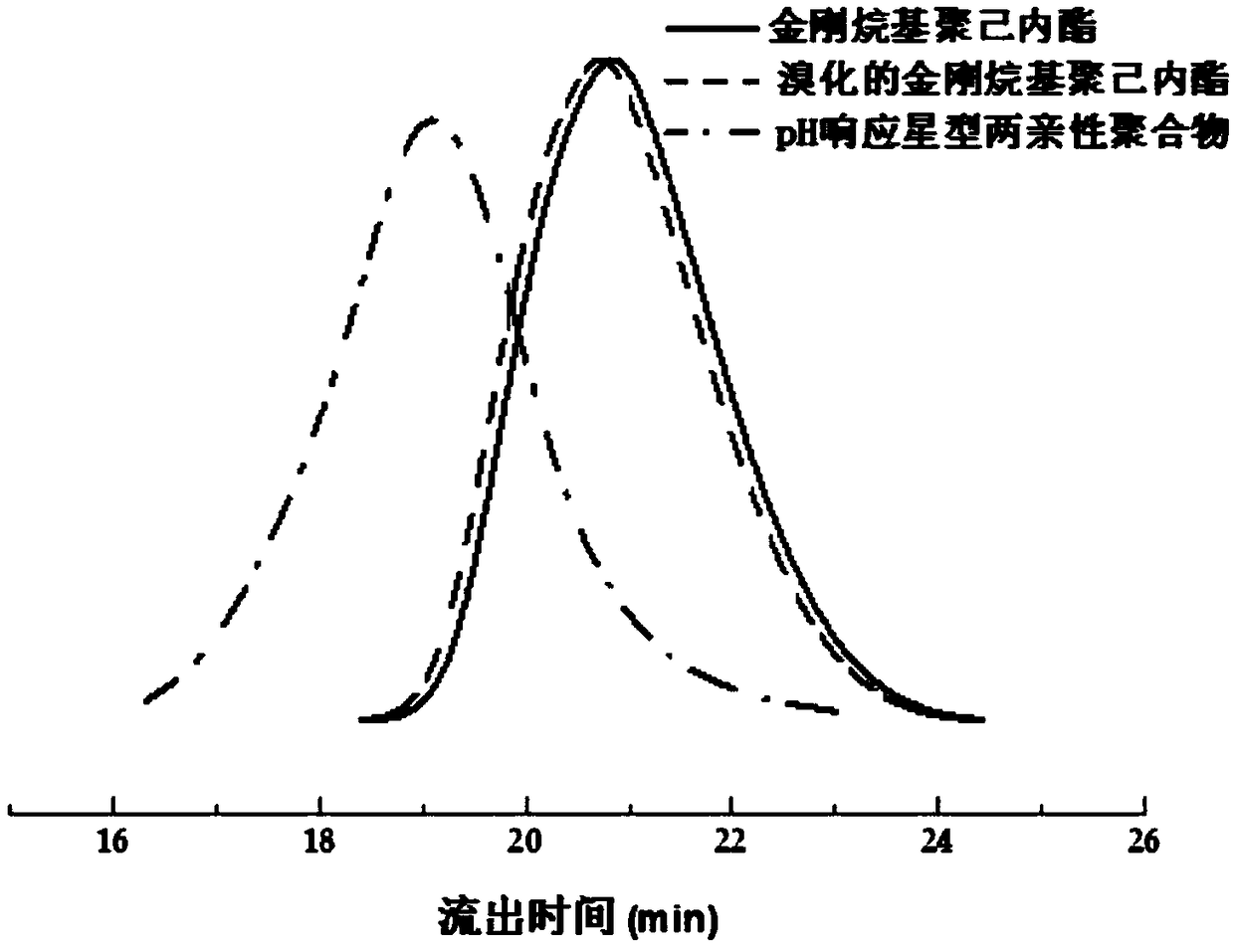
[0035] Such as figure 1 As shown, the synthetic steps of the present embodiment include:
[0036] (1) Synthesis of adamantyl polycaprolactone
[0037] Add 1,3,5,7-tetrahydroxyadamantane (0.04g, 0.2mmol) into the Shrek reaction flask containing a stirring bar, and after pumping air and argon for 3 times, the hydrophobic monomer ε-hexyl Lactone (9.12g, 8mmol), the first catalyst stannous octoate (0.009g, 0.022mmol) and the first reaction solvent anhydrous toluene (30mL) were added into the reaction flask through a syringe, and after ultrasonication for 20min, the reaction was carried out at 90°C for 36h to obtain Crude product containing adamantyl polycaprolactone.
[0038] In order to obtain pure adamantyl polycaprolactone, after the reaction is completed, the excess first reaction solvent toluene in the crude product is removed by rotary evaporation; dissolved with the second solvent tetrahydrofuran, and slowly added dropwise to 10 times the first In the precipitating agent...
Embodiment 2
[0049] (1) Synthesis of adamantyl polycaprolactone
[0050] Add 1,3,5,7-tetrahydroxyadamantane (0.04g, 0.2mmol) into the Shrek reaction flask containing a stirring bar, pump air-argon in this way for 3 times, and the hydrophobic monomer ε-hexene Ester (28.5g, 25mmol), the first catalyst zinc acetate (0.029g, 0.155mmol) and the first reaction solvent anhydrous xylene (30mL) were added into the reaction flask through a syringe, and after ultrasonication for 20min, they were reacted at 100°C for 12h to obtain Crude product of adamantyl polycaprolactone.
[0051] In order to obtain pure adamantyl polycaprolactone, after the reaction is completed, the redundant first reaction solvent xylene in the crude product is removed by rotary evaporation; dissolved with the second solvent tetrahydrofuran, and then slowly added dropwise to 10 times the amount A precipitating agent, ether, filtered the precipitated solid, and repeated this operation 2-3 times to obtain adamantyl polycaprolacto...
Embodiment 3
[0059] (1) Synthesis of adamantyl polycaprolactone
[0060] Add 1,3,5,7-tetrahydroxyadamantane (0.04g, 0.2mmol) into the Shrek reaction flask containing a stirring bar, and after pumping air and argon for 3 times, the hydrophobic monomer ε-hexyl Lactone (42.75g, 37.5mmol), the first catalyst aluminum isopropoxide (0.086g, 0.419mmol) and the first reaction solvent anhydrous toluene (30mL) were added to the reaction bottle through a syringe, and after ultrasonication for 20min, the reaction was carried out at 130°C for 48h , to obtain a crude product containing adamantyl polycaprolactone.
[0061] In order to obtain pure adamantyl polycaprolactone, after the reaction is finished, the excess first reaction solvent toluene in the crude product is removed by rotary evaporation; dissolved with the second solvent methylene chloride, and then slowly added dropwise to 10 times In methanol as the first precipitant, filter the precipitated solid, and repeat this operation 2 to 3 times; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com