Antimicrobial fusion peptide as well as preparation method and application thereof
A technology that fuses peptides and peptide chains, applied in the field of antibacterial drugs, can solve problems such as lack of antibacterial activity and anticancer drugs that cannot inhibit bacterial growth, and achieve the effect of improving anticancer effects and reducing drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
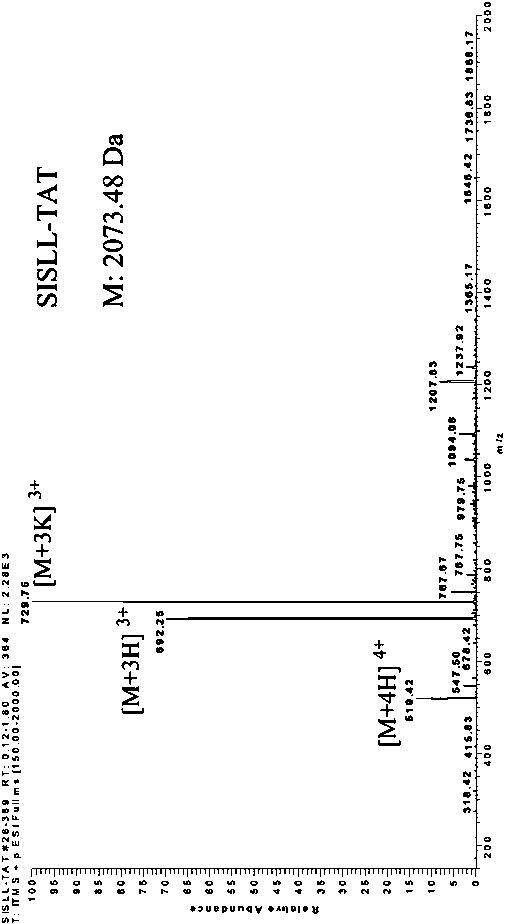
[0026] Such as Figure 1 to Figure 3 As shown, the present invention provides an antibacterial fusion peptide, which is SISLL-TAT in which the nitrogen terminal of TAT (47-57) is linked to BRCA1 (782-786), and its peptide chain sequence is: SISLLYGRKKRRQRRR.
Embodiment 2
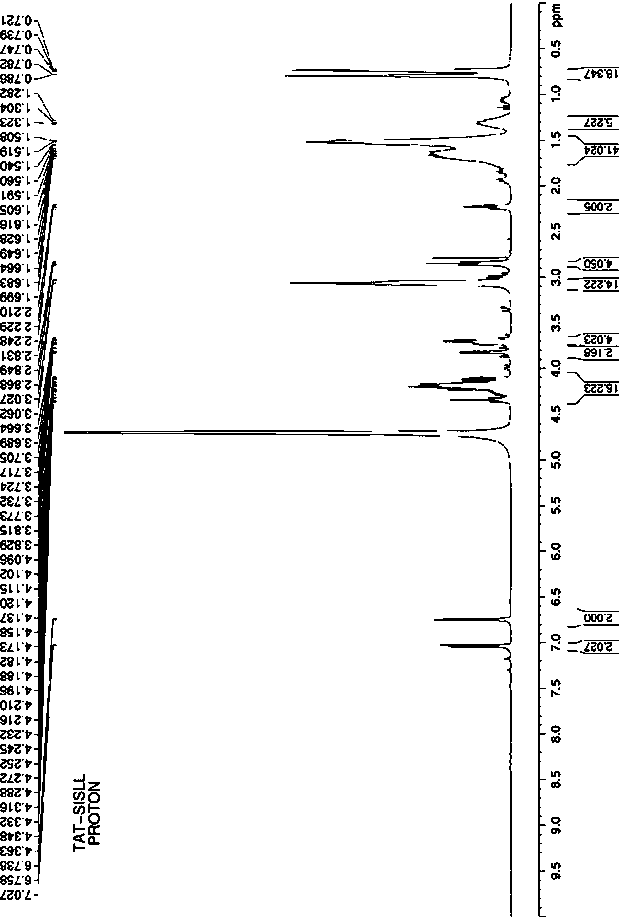
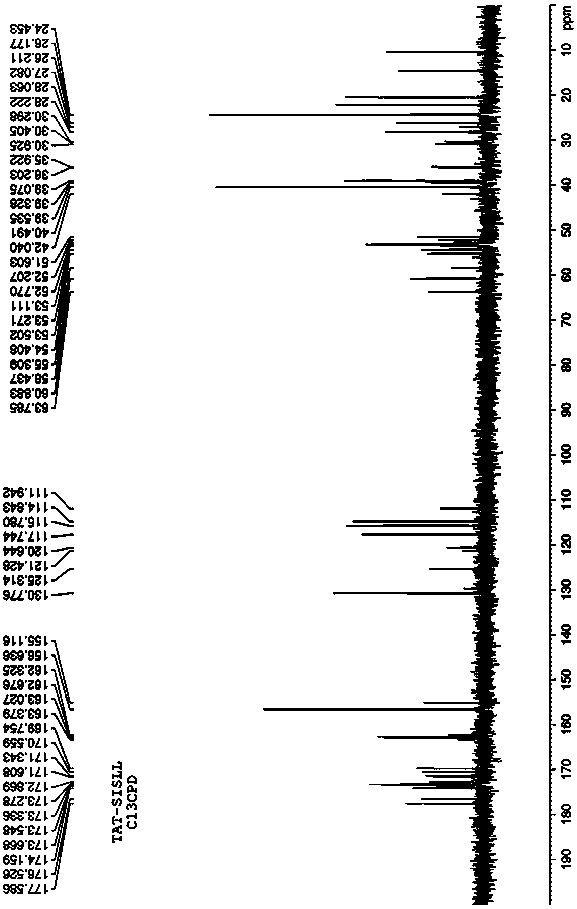
[0028] Such as Figure 4 to Figure 6 As shown, the present invention provides an antibacterial fusion peptide. The peptide sequence of the antibacterial fusion peptide is TAT-SISLL with the carbon terminal of TAT (47-57) connected to BRCA1 (782-786), and its peptide chain sequence is: YGRKKRRQRRRSISLL.
[0029] The present invention also provides the preparation method of antibacterial fusion peptide in embodiment one and embodiment two, and this method is:
[0030] Using Wang resin as the carrier, Fmoc as the amino acid side chain protecting group, piperidine in DMF solution as the deprotection reagent, HBTU, HOBT and DIEA as the amino acid condensing agent, according to the sequence of the target antibacterial fusion peptide, from the carbon (C-) terminal Go to the nitrogen (N-) terminal to couple the corresponding amino acid; until the coupling of the nitrogen-terminal amino acid is completed, remove the Fmoc protecting group of the nitrogen-terminal amino acid, wash and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com