Method for preparing dovitinib intermediate with microchannel reaction device
A technology of microchannel reaction and dovitinib, applied in organic chemistry and other directions, can solve problems such as low product yield, and achieve the effect of reducing consumption and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
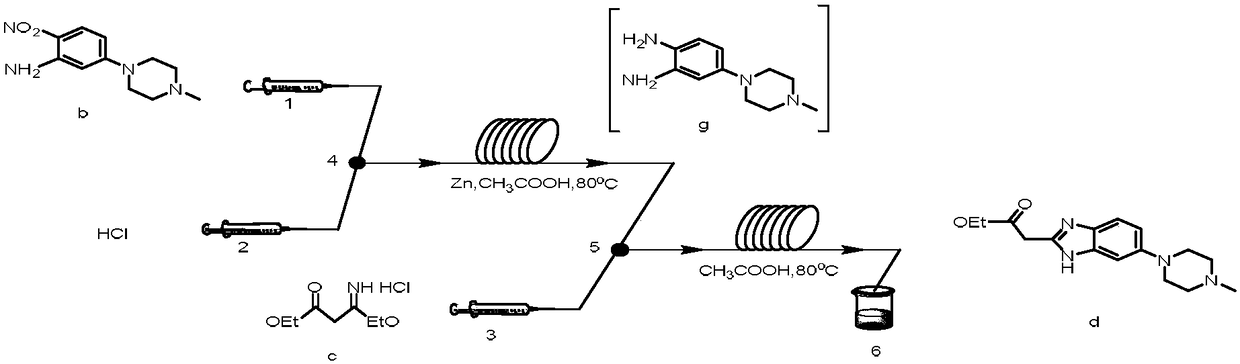
[0033] (1) Synthesis of 5-(4-methylpiperazinyl-1-yl)-2-nitroaniline (compound b)
[0034]
[0035] a, adding ethanol to the reaction solution for reaction
[0036]Under nitrogen protection, add compound a (20g, 0.12mol) and N-methylpiperazine (50g, 0.50mol) into a 100mL three-necked flask, then add 75mL of ethanol, stir, heat the oil bath to reflux, and react for 24h , use thin layer chromatography to track whether a is completely reacted, if not, continue to react until a is completely reacted. Pour the reaction solution into a 1L beaker containing 400mL of water while it was hot. A yellow substance precipitated out. The suspension was mechanically stirred for half an hour, filtered with suction, and the filter cake was washed 2 to 3 times with ice-cold ethanol. The filtrate was discarded, and the obtained filter cake was dried in an oven at 60° C. to obtain the product compound b with a yield of 65%.
[0037] b. Do not add ethanol to the reaction solution
[0038] Unde...
Embodiment 2
[0049] The preparation method is the same as in Example 1, and step (1) adopts the b method of step (1) in Example (1), but the mol ratio of 5-chloro-2-nitroaniline to N-methylpiperazine is 1 : 2, the productive rate of 5-(4-methylpiperazinyl-1-yl)-2-nitroaniline is 79%;
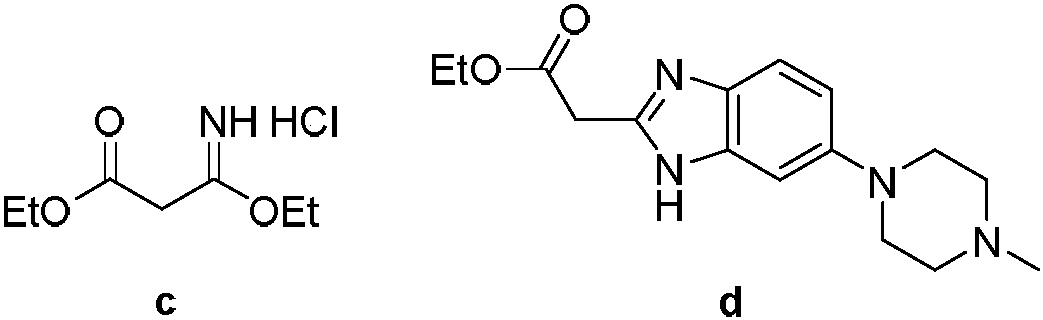
[0050] Step (2) adopts the b method of step (2) in the embodiment (1), but 5-(4-methylpiperazinyl-1-yl)-2-nitroaniline and β-ethoxyl-β The molar ratio of ethyl iminopropionate hydrochloride is 1:1.2 to obtain ethyl-2-(6-(4-methylpiperazin-1-yl)benzimidazol-2-yl)ethyl acetate The yield was 82%;
[0051] Moles of ethyl-2-(6-(4-methylpiperazin-1-yl)benzimidazol-2-yl)ethyl acetate, 2-amino-6-fluorobenzonitrile, KHMDS in step (3) The ratio was 1:1.2:3, and the yield of dovitinib was 69%.
Embodiment 3
[0053] The preparation method is the same as in Example 1, and step (1) adopts the b method of step (1) in Example (1), but the mol ratio of 5-chloro-2-nitroaniline to N-methylpiperazine is 1 : 6, the productive rate of 5-(4-methylpiperazinyl-1-yl)-2-nitroaniline is 85%;
[0054] Step (2) adopts the b method of step (2) in the embodiment (1), but 5-(4-methylpiperazinyl-1-yl)-2-nitroaniline and β-ethoxyl-β The molar ratio of ethyl iminopropionate hydrochloride is 1:3 to obtain ethyl-2-(6-(4-methylpiperazin-1-yl)benzimidazol-2-yl)ethyl acetate The yield was 87%;
[0055] Moles of ethyl-2-(6-(4-methylpiperazin-1-yl)benzimidazol-2-yl)ethyl acetate, 2-amino-6-fluorobenzonitrile, KHMDS in step (3) The ratio was 1:1.5:4, and the yield of dovitinib was 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com