Application of amino acid ester compounds in preparation of anti-CVB3 (anti-coxsackie B virus 3) drugs
A technology of amino acid esters and compounds, which is applied in the field of application of amino acid ester compounds in the preparation of anti-CVB3 virus drugs, and can solve the problems of unreported inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
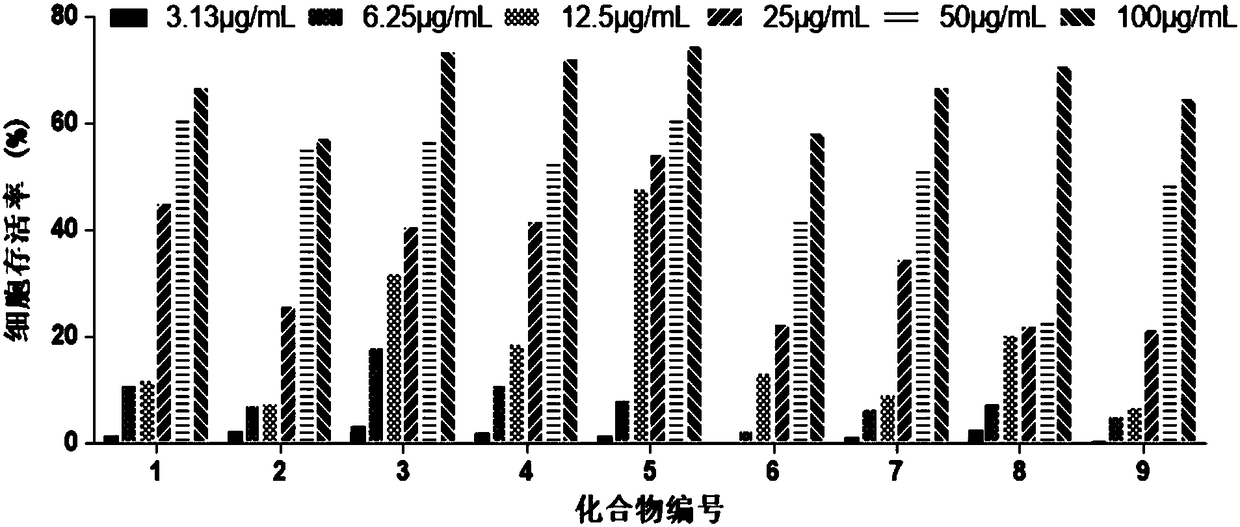
[0020] Embodiment 1 Amino acid ester compound anti-CVB3 activity analysis
[0021] Amino acid ester compounds tested include glycine ethyl ester (1), L-leucine methyl ester (2), L-alanine methyl ester (3), L-alanine tert-butyl ester (4), β-propanine Ethyl Alanate (5), D-Leucine Methyl Ester (6), Glycine tert-Butyl Ester (7), β-Alanine tert-Butyl Ester (8), L-Leucine tert-Butyl Ester (9) .
[0022] (1) Toxicity of compounds to host Hep-2 cells
[0023] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the incubator was cultured to cover the monolayer, the cell culture medium was discarded, and the cell maintenance medium containing different concentrations of the test compound (ribavirin as a positive control drug) was added to continue the culture. After 48 hours, the cytotoxicity was visually observed under a microscope and recorded respectively. Cell viability was determined by MTT method. The specific steps of the MTT method are as follows: add 30 μL of M...
Embodiment 2
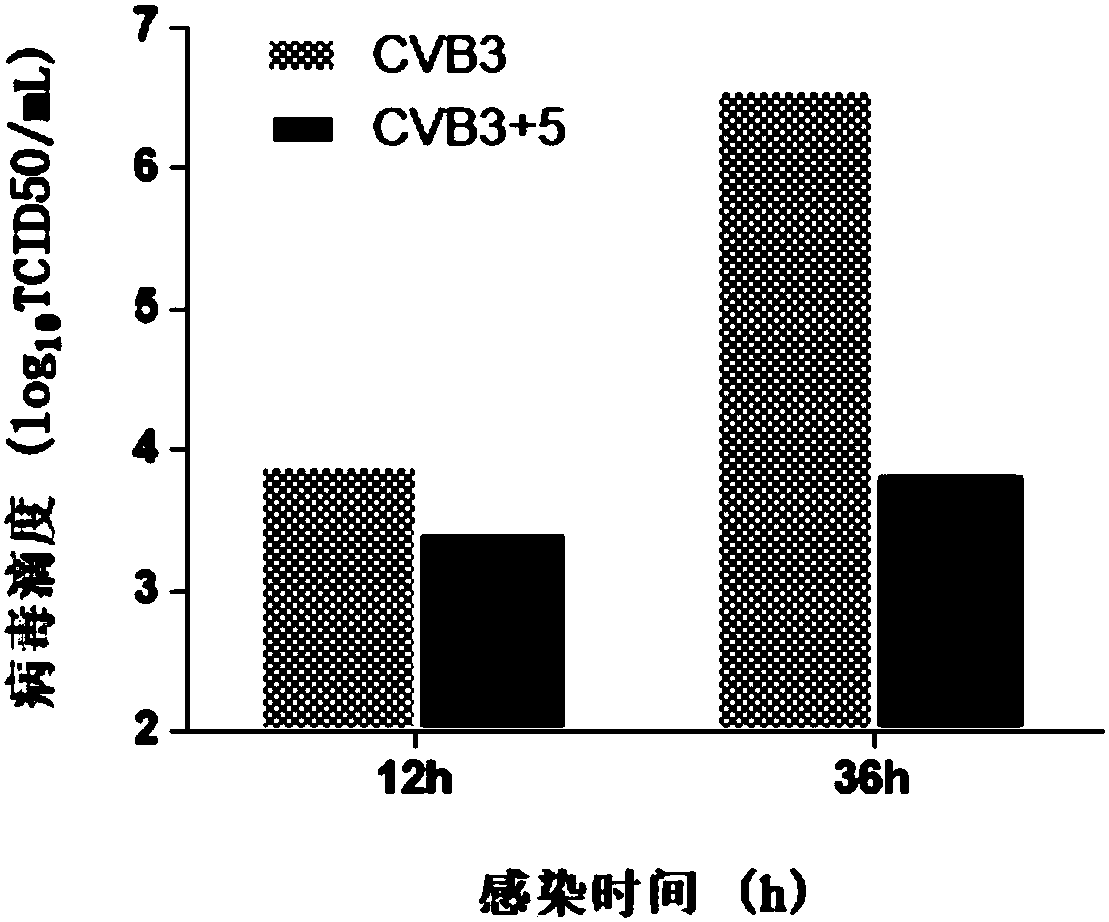
[0040] Example 2 Compound 5 (beta-alanine ethyl ester) inhibits the production of CVB3 progeny virus
[0041] Hep-2 cells in the logarithmic growth phase were plated in 96-well plates, and 100TCID after the monolayer was overgrown 50 CVB3 infected cells, incubated at 37°C for 1.5h, removed the virus solution, washed three times with PBS, and added cell maintenance solution containing 50 μg / mL compound 5 (β-alanine ethyl ester). Cells and supernatant culture fluid were collected at 12h and 36h respectively, and after three freeze-thaw lysis at -20°C and 37°C, TCID 50 Methods To determine the titer of CVB3 virus.
[0042] The result is as image 3 As shown, after 12 hours of virus infection, obvious progeny virus titers can be detected, and after 36 hours of infection, the progeny virus titers showed a substantial increase. Compared with the virus control group, the Hep-2 cells treated with 50 μg / mL No. 5 compound showed a certain inhibitory effect at 12 hours, and showed a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com