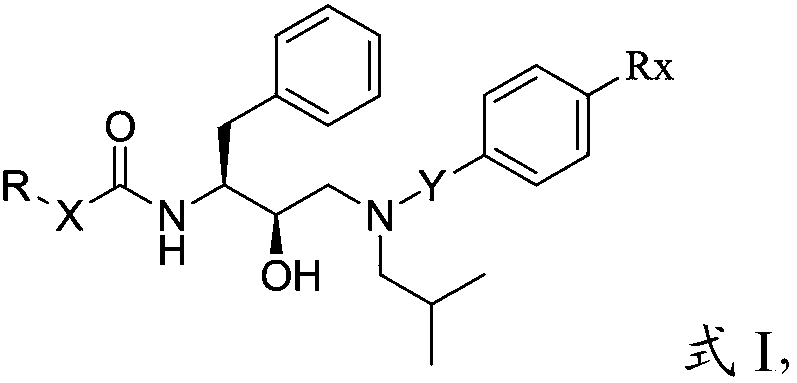
Amide derivative or pharmaceutically acceptable salt thereof and preparation method and application thereof
A technology of derivatives and amides, applied in the field of amide derivatives and their preparation, can solve the problems of AIDS derivatives with HIV-1 protease activity and reverse transcriptase activity that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] The invention provides a preparation method of the amide derivatives, comprising the following steps:
[0097] The present invention also provides a preparation method of the amide derivatives, comprising the following steps:
[0098] 1) When Rx is methoxy, methylthio, nitro or aminomethyl in the compound of structure shown in formula I:
[0099] When X is -CH 2 - or without any substituting group, when R is Ra, Rb, Rc, Rd or Rg:
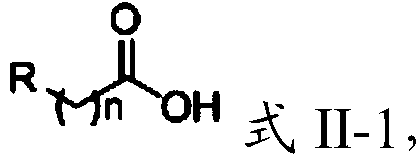
[0100] Condensing the compound having the structure shown in formula II-1 with the amine derivative under the action of a catalyst to obtain the compound having the structure shown in formula I;
[0101]
[0102] In formula II-1, n=0 or 1;
[0103] The Ra is The Rb is The Rc is The Rd is The Rg is
[0104] Among them, M 1 C or N, M 2 O or S; L 1 C or N, L 2 is O or S; R 1 ~R 7 independently hydrogen, hydroxyl, hydroxymethyl, amino, halogen, carbonyl, C1-C6 alkyl, C3-C6 cycloalkyl, C2-C6 alkenyl, C3-C6 cycloalkenyl, C1...
Embodiment 1
[0235] 1. Synthesis of N-((2R,3S)-2-hydroxy-3-amino-4-phenylbutane)-N-isobutyl-4-methoxybenzenesulfonamide (intermediate III1a):
[0236] 1), Synthesis of (1S,2R)-1-benzyl-2-hydroxyl-3-(isobutylamine)carbamate tert-butyl ester (intermediate 2):
[0237]
[0238](S)-1-((S)-Oxiran-2-yl)-2-phenylethylcarbamate tert-butyl ester (starting material 1, 75.94 mmol), acetonitrile 80 mL and isobutylamine (189.46 mmol) were added Transfer to a 200mL eggplant-shaped bottle, and stir the reaction at 80°C for 5 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, and concentrated under reduced pressure to remove the solvent. The crude product was recrystallized from a mixture of ethyl acetate and n-hexane with a mass ratio of 1:9 to obtain a white product, Intermediate 2 (21.2 g, 83%). LC-MS of intermediate 2 (ESI, M+H + ) m / z 337.2.
[0239] 2), Synthesis of (1S,2R)-1-benzyl-2-hydroxy-3-(N-isobutylamine-4-methoxyphenylsulfonamide) tert-butyl c...
Embodiment 2
[0250] 1. Preparation of N-((2R,3S)-2-hydroxyl-3-amino-4-phenylbutane)-N-isobutyl-4-nitrobenzenesulfonamide (intermediate III1b) synthesis
[0251] The synthesis of intermediate III1b is similar to the synthesis of intermediate III1a in Example 1, the only difference is that 4-methoxybenzenesulfonyl chloride is replaced by 4-nitrobenzenesulfonyl chloride.
[0252] 2. 4-Hydroxy-3,5-dimethoxyphenyl-N-[(2S,3R)-3-hydroxy-4-(N-isobutyl-4-nitrophenylsulfonamide)- Synthesis of 1-phenylbutyl-2-yl]-carboxamide (intermediate 12)
[0253]
[0254] The synthesis of intermediate 12 is similar to the synthesis of compound 1 in Example 1, the only difference is that raw material 10 is replaced by 3,5-dimethoxy-4-hydroxybenzoic acid (raw material 11), and intermediate III1a is replaced by intermediate Body III1b, the reaction gave a white powder solid, namely Intermediate 12 (0.16.g, 52%), the LC-MS of Intermediate 12 (ESI, M+H + ) m / z 602.5.
[0255] 3. 4-Hydroxy-3,5-dimethoxyphenyl-N-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com