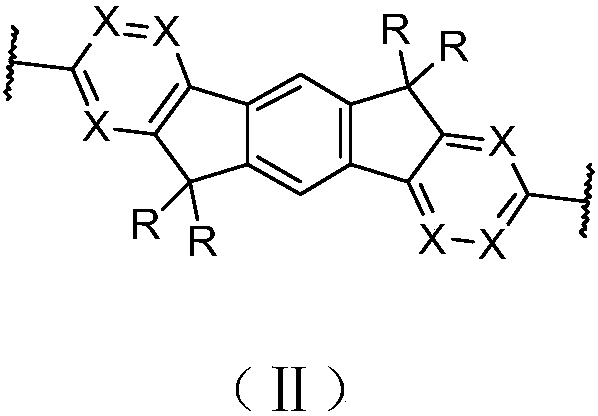
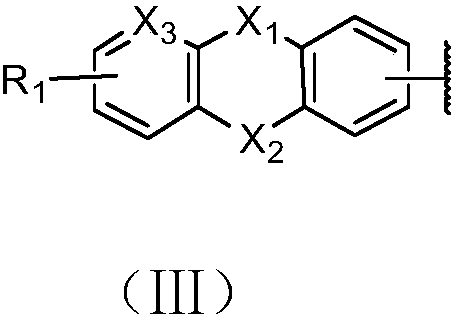
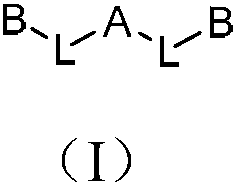Polyfluorene derivative and organic light-emitting device thereof
A technology of electroluminescent devices and derivatives, which is applied in the direction of electric solid-state devices, electrical components, luminescent materials, etc., and can solve the problems of short life of organic light-emitting devices, reduced material usage, poor thermal stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Preparation of compound 2
[0087]
[0088] Preparation of Intermediate A
[0089] Add 2-1 (31.81g, 100.00mmol) into 50.0mL of concentrated sulfuric acid, stir at room temperature for 2h, find that the color of the reaction solution turns yellow, slowly add 300.0mL of water, and then neutralize the reaction solution with sodium hydroxide to neutral, with solid Precipitated, cooled and filtered to obtain solid 1-2 (21.18g, 75.56mmol). 2-2 was dissolved in 150ml of ethylene glycol, 40ml of 30% hydrazine hydrate was added to reflux reaction, the reaction liquid was cooled and poured into water, and the precipitate was filtered to obtain white solid 2-3 (14.29g, 56.12mmol). Dissolve 1-3 in 150mL toluene, add 30g 2-4, 1.5g tetrabutylammonium bromide, reflux, cool and filter column chromatography to obtain white powder (14.76g, 47.55mmol). Finally, it was brominated, and the white powder was dissolved in 100.0mL of chloroform, and 1.0g of anhydrous ferric chloride was ad...
Embodiment 2
[0095] Preparation of Compound 22
[0096]
[0097] Preparation of Compound 22
[0098] Compound 22 was obtained by replacing 2-4 in Example 1 with 22-4 shown above, and 2-6 with 22-6 shown above. Mass Spectrum m / z: 1041.31 (calculated: 1041.32). Theoretical element content (%)C 80 h 52 N 2 : C, 92.28; H, 5.03; N, 2.69 Measured element content (%): C, 92.27; H, 5.02; N, 2.68 The above confirmed that the obtained product is the target product 22.
Embodiment 3
[0100] Preparation of compound 39
[0101]
[0102] Preparation of compound 39-6
[0103] The same as the preparation of compound 2-6 in Example 1.
[0104] Preparation of compound 39
[0105] Compound 39 was obtained by replacing 2-4 in Example 1 with 39-4 shown above, and 2-6 with 39-6 shown above. Mass Spectrum m / z: 915.11 (calculated: 915.12). Theoretical element content (%)C 68 h 42 N 4 : C, 89.25; H, 4.63; N, 6.12 The measured element content (%): C, 89.26; H, 4.64; N, 6.13. It was confirmed above that the obtained product was the target product 39.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com