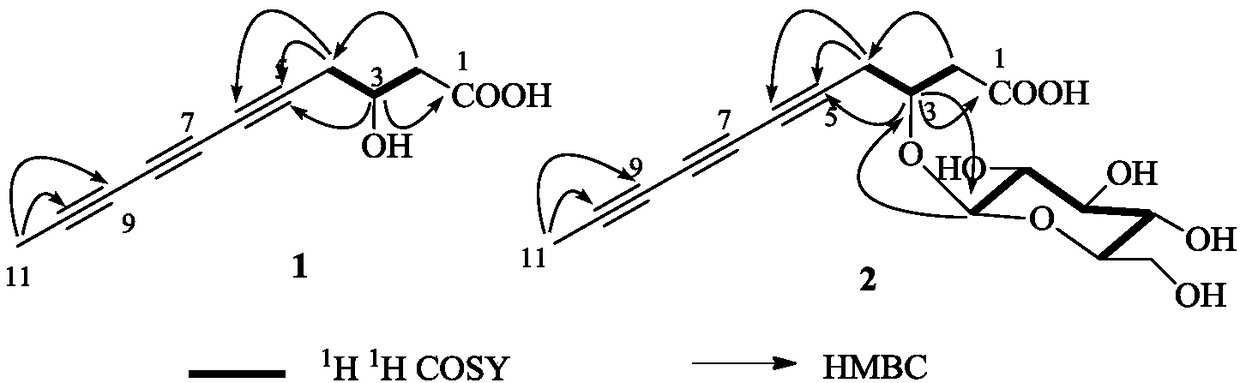
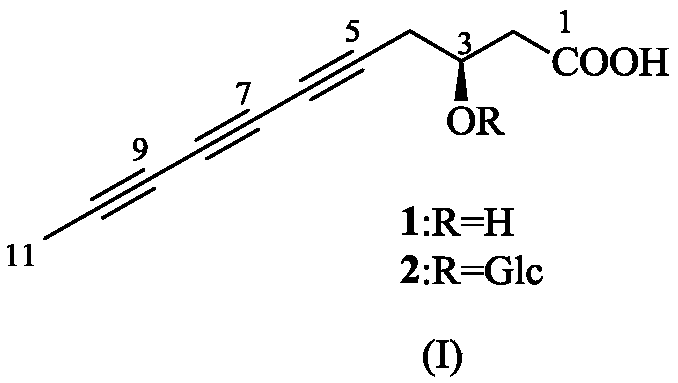
Capillary wormwood herb triynoic acid compound, pharmaceutical composition thereof and application thereof
A compound and composition technology, applied in the separation/purification of carboxylic acid compounds, active ingredients of anhydride/acid/halide, chemical instruments and methods, etc., can solve the application report of drugs for hepatitis B virus without compound pharmaceutical composition , Few studies on anti-HBV activity, and no reports of pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of Compounds 1 and 2:
[0055] Extraction and separation of compounds 1 and 2:
[0056] Take the dried whole herb of Artemisia capriculum or Artemisia chinensis, crush it, and extract twice with 90% ethanol under reflux, each time for 3 hours, combine the ethanol extracts, recover the ethanol under reduced pressure to obtain the extract. The extract is dissolved and adsorbed on silica gel with 80% ethanol, placed at room temperature to evaporate the solvent, ground and sieved, and subjected to silica gel column chromatography, using 9:1:0, 9:1:0.1, 8:2:0.2, 6: 4:0.4 chloroform-methanol-water gradient elution, to obtain 5 components A-E, component B was prepared by MCI CHP-20Pgel column medium pressure, 20:80 to 80:20 methanol-water gradient elution, to obtain 4 Fractions B-1~B-4, B-3 were eluted with 9:1 ethyl acetate-methanol through silica gel column chromatography to obtain two fractions B-3-1 and B-3-2, B-3-2 via Rp-C 18 The column was prepared under ...
Embodiment 2
[0083] Compound Combination Pharmaceutical Form - Tablet:
[0084] Compound 1 or / and 2 of the present invention is used as the preparation of the drug combination tablet of active ingredient: use compound 1 or / and 2 as drug active ingredient, use the excipient described in Table 3 as the adjuvant component of preparation combination drug tablet, Tablet samples containing 5 to 60 mg of the pharmaceutical ingredients of compound 1 or / and 2 were made according to the ratio. Table 3 shows the formula ratio of common tablets:
[0085] Table 3 The raw material and adjuvant formula of compound 1 or / and 2 combination medicine tablet of the present invention
[0086]
[0087]
[0088] The method of preparing a certain amount of compound 1 or / and 2 with excipients and auxiliary materials into tablet preparations of different doses is to uniformly mix several excipients and auxiliary materials with the raw material drug, and add 1% hydroxymethylcellulose sodium solution in an appro...
Embodiment 3
[0090] Compound Combination Pharmaceutical Form - Capsules:
[0091] Preparation of pharmaceutical combination capsule preparations containing compound 1 or / and 2 as active ingredients: use compound 1 or / and 2 samples as pharmaceutical active ingredients, use several excipients in the table as excipients for preparing combination drug capsules, according to the proportion Proportioning is made into capsule preparations containing 5-50 mg of the pharmaceutical ingredients of Compound 1 or / and 2 in each capsule, and Table 4 provides the formula ratio of common capsule preparations:
[0092] Table 4 The bulk drug and adjuvant formula of the compound drug capsule preparation
[0093]
[0094] The method for preparing a certain amount of compound 1 or / and 2 samples and excipients and auxiliary materials into capsule preparations is: mix several excipients and auxiliary materials with compound 1 or / and 2 evenly, add 1% hydroxymethyl cellulose Appropriate amount of sodium solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com