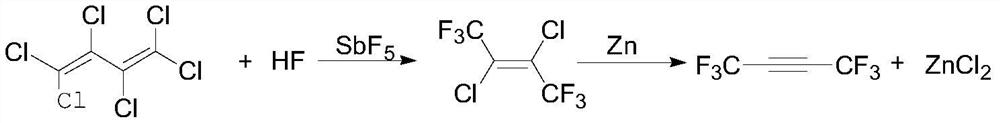
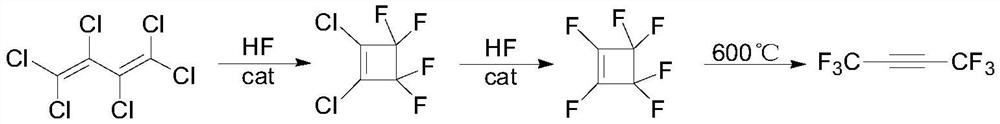
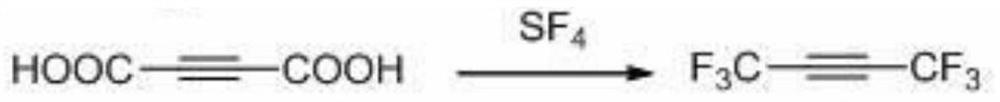A kind of preparation method of hexafluoro-2-butyne
A technology of butyne and hexachlorobutadiene is applied in the field of preparation of hexafluoro-2-butyne, and can solve the problems of difficulty in obtaining 2-butyne diacid, high raw material price of sulfur tetrafluoride, unsuitability for industrialization and the like , to achieve the effect of low cost, less three waste emissions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 2L stainless steel reaction kettle with a condenser, add 260g of hexachlorobutadiene, 780g of sulfolane, and 348g of potassium fluoride, raise the temperature to 100°C for reaction, collect the gas phase reaction products, wash with water, wash with alkali, and rectify to obtain hexafluoro- 2-butyne product, the product is sampled and analyzed by gas chromatography, the conversion rate of raw material hexachlorobutadiene is 99%, and the product selectivity is 96%.
Embodiment 2
[0036] Add 260g of hexachlorobutadiene, 1,300g of diethylene glycol dimethyl ether, and 710g of chromium fluoride into a 2L stainless steel reaction kettle with a condenser, heat up to 250°C for reaction, and collect the gaseous reaction products and wash them with water and alkali 1. The hexafluoro-2-butyne product was obtained by rectification, and the product was sampled for gas chromatography analysis. The conversion rate of raw material hexachlorobutadiene was 99%, and the product selectivity was 95%.
Embodiment 3
[0038] Add 260g of hexachlorobutadiene, 800g of N-methylpyrrolidone, and 610g of zinc fluoride into a 2L stainless steel reaction kettle with a condenser, heat up to 150°C for reaction, collect the gas phase reaction products and wash with water, alkali, and rectify The hexafluoro-2-butyne product was obtained, and the product was sampled for gas chromatography analysis. The conversion rate of raw material hexachlorobutadiene was 99%, and the product selectivity was 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com