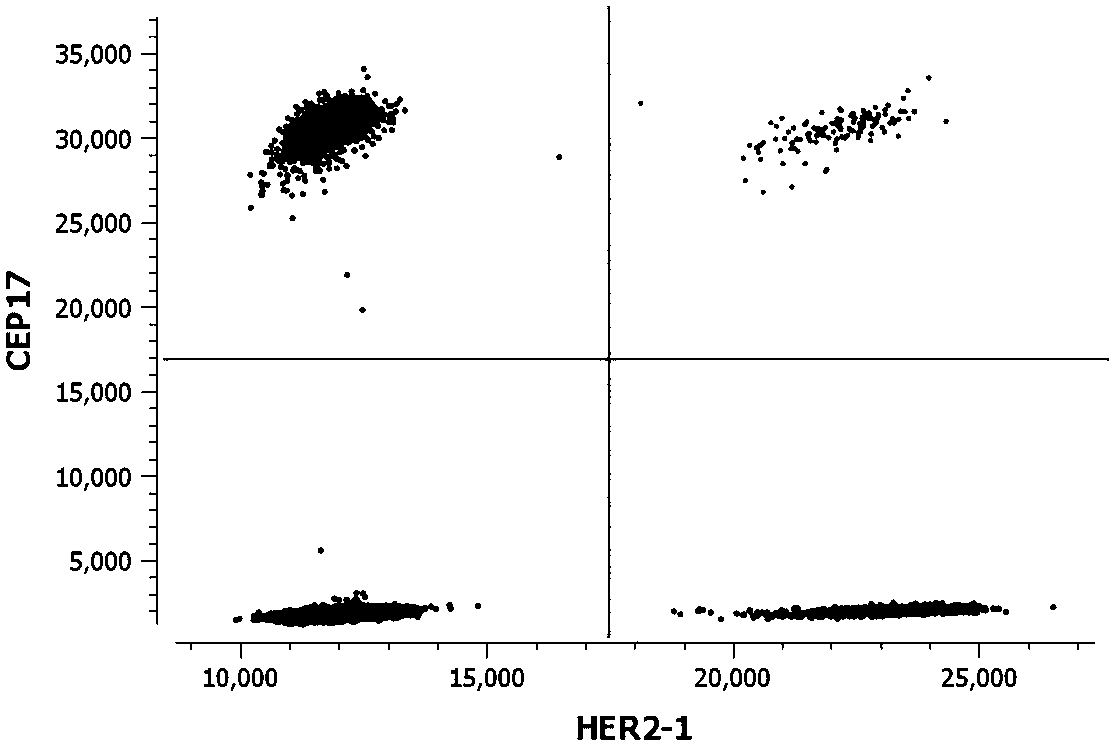
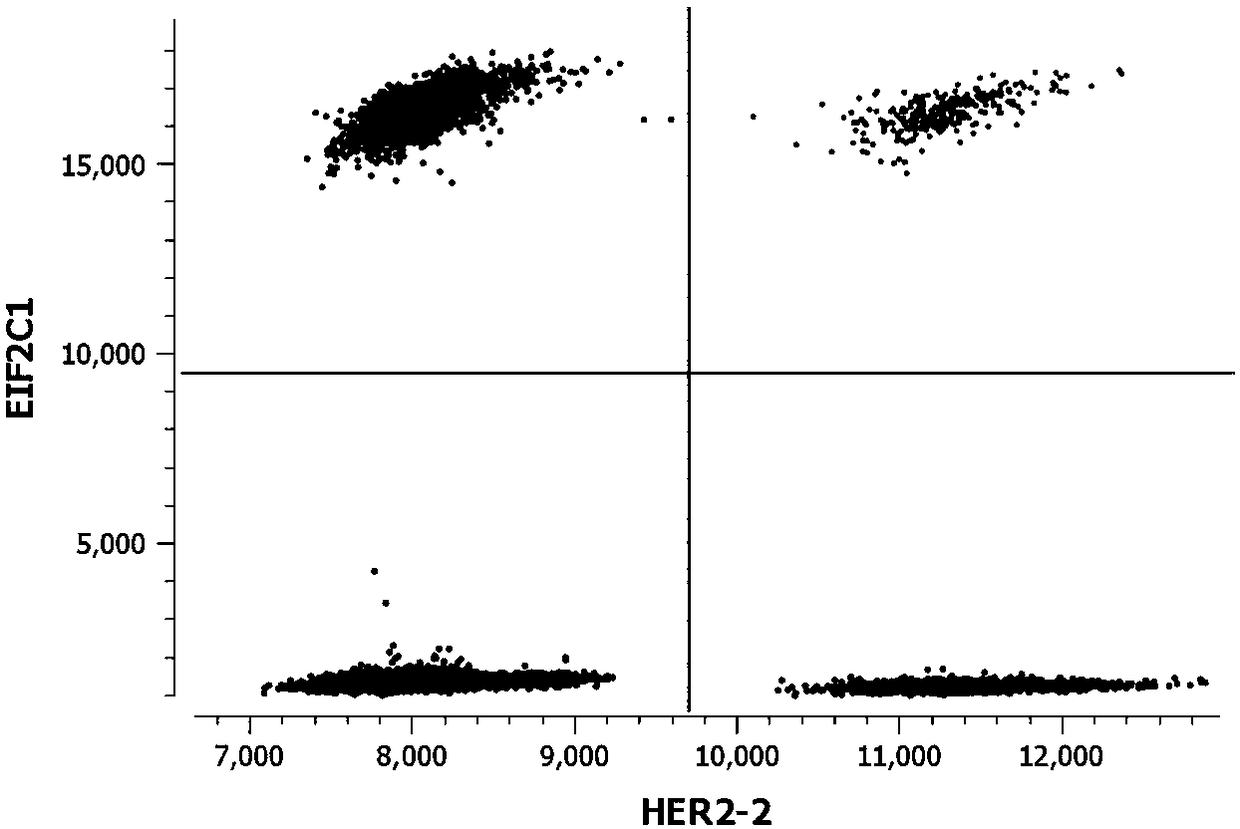
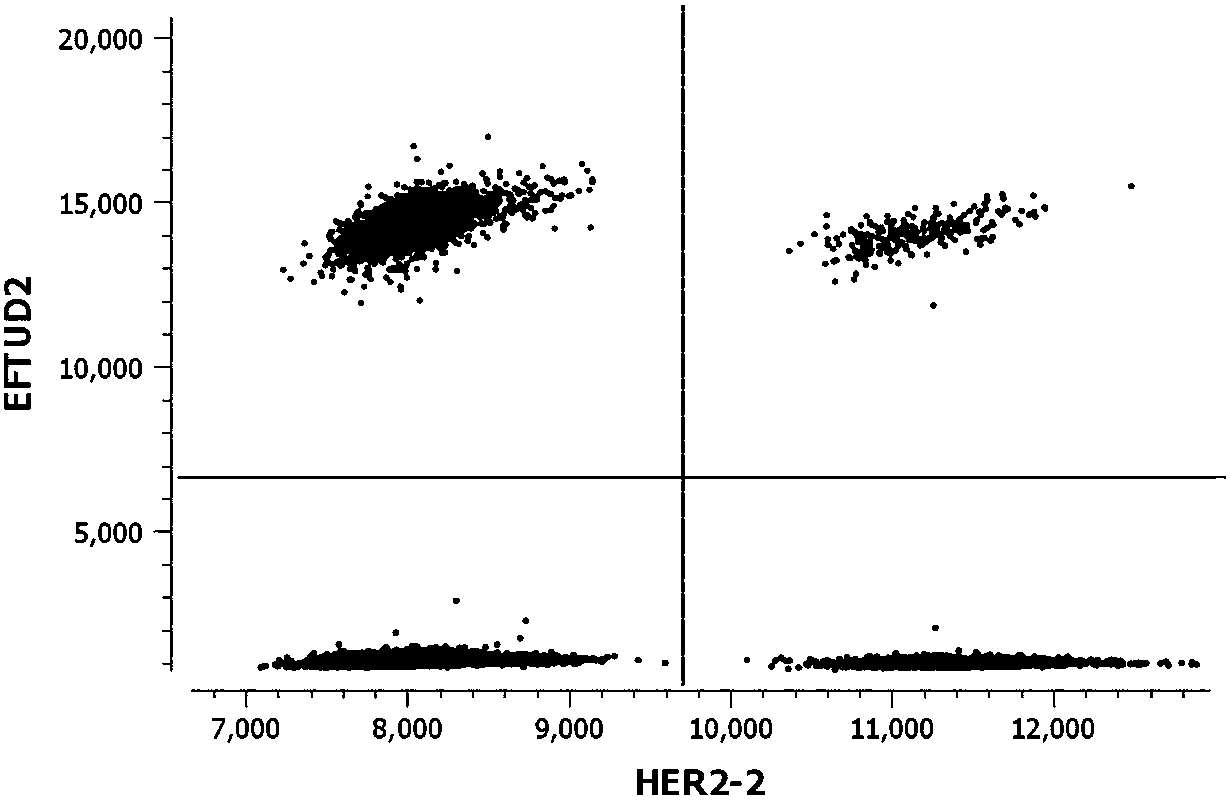
Kit for detecting copy number variation of HER-2 gene by virtue of digital PCR technique and method
A HER-2, gene copy number technology, applied in the field of biomedical nucleic acid detection, can solve problems such as low accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Design of specific PCR primers and detection probe sequences for detecting HER-2 gene copy number variation
[0032] 1. Sequence acquisition: See Table 1 for the detection sites of HER-2, CEP17, EFTUD2, and EIF2C1 genes involved in the present invention.
[0033] Table 1, HER-2, CEP17, EFTUD2, EIF2C1 gene detection sites
[0034] Gene
[0035] 2. Design method: According to the HER-2, CEP17, EIF2C1, and EFTUD2 gene sequences published in the NCBI database, specific primers and detection probes were designed (Table 2).
[0036] Table 2, HER-2, CEP17, EIF2C1, EFTUD2 primer and detection probe sequence list
[0037] Gene loci
[0038] 3. Design primers and detection probes based on the specific sequences obtained above. The primers and detection probes used in the patent of the present invention were synthesized at Thermo Fisher Scientific Corporation (Shanghai), USA.
Embodiment 2
[0039] Example 2: Using a kit and method for detecting HER-2 gene copy number variation based on digital PCR technology to detect HER-2 gene copy number variation in paraffin tissue section samples.
[0040] 1. Materials: Paraffin section samples of 18 breast cancer patients who have been identified by IHC or FISH.
[0041] 2. Method: use Naica Crystal Digital TM Digital PCR instrument (French STILLA Technologies company) carries out digital PCR detection.
[0042] (1) Nucleic acid extraction: It is recommended to use paraffin section DNA extraction kit (QIAGEN, USA, CatNo. 56404) for DNA extraction. The extraction step was carried out according to the product instructions, and 50 μL of DNA solution was collected and directly tested or stored at -20°C. At the same time, negative and positive quality control products were tested.
[0043] (2) The digital PCR reaction solution was prepared according to Table 3. After the preparation was completed, the PCR reaction tube was vo...
Embodiment 3
[0063] Example 3: Using a kit and method for detecting HER-2 gene copy number variation based on digital PCR technology to detect HER-2 gene copy number variation in blood samples.
[0064] 1. Materials: Randomly select blood samples from 5 of the 18 breast cancer patients in Example 2
[0065] 2. Method: use Naica Crystal Digital TM A digital PCR instrument (French STILLA Technologies company) was used for detection.
[0066] (1) Sample collection: blood samples are collected by venipuncture, and 10 mL of blood samples are collected using STRECK BCT tubes or EDTA anticoagulant tubes (purple); if plasma separation cannot be performed within 2 hours, a protective tube containing free DNA is required when collecting whole blood. Special room temperature blood collection tubes (such as Streck BCT tubes) with agents and anti-cell lysis protection agents.
[0067] (2) Preservation and transportation of blood samples: STRECK BCT tubes belong to the blood collection tubes used for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com