Modified human lactoferrin gene suitable for bombyx mori silk gland expression as well as expression system adopting modified human lactoferrin gene and applications
A technology of human lactoferrin and expression system, which is applied in the field of expression system for expressing human lactoferrin gene, to achieve the effect of controlling the risk of disease transmission and huge market development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, transformation human lactoferrin gene synthesis
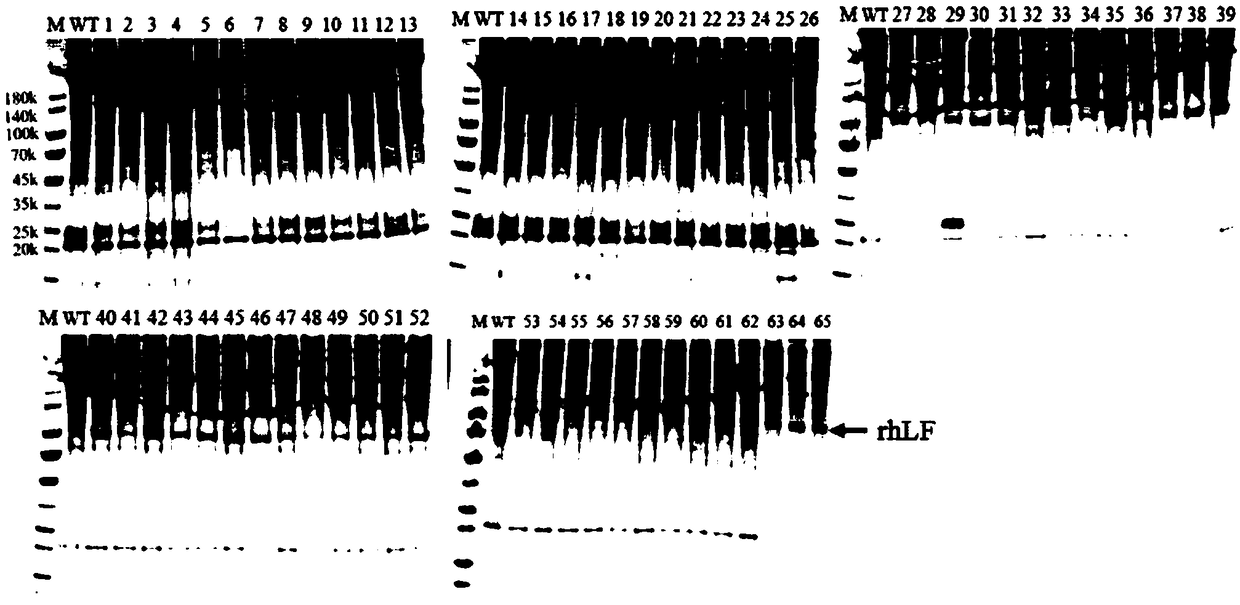
[0031] Download the amino acid sequence of the mature peptide of human lactoferrin (Human lactoferin, hLF, GenBank: M93150.1) from NCBI, and optimize the coding sequence according to the silkworm codon usage preference. As shown, the amino acid sequence is shown in the 1st to 700th positions of SEQ ID NO.2, and the gene sequence was synthesized by the company. Six histidine amino acids were fused to the C-terminus of the human lactoferrin (hLF) amino acid sequence to form hLF-his6. Both ends of the two synthetic recombinant protein gene sequences are respectively connected with BamHI and NotI restriction sites, as shown in SEQ ID NO.1, and the encoded amino acid sequence is shown in SEQ ID NO.2.
Embodiment 2
[0032] Embodiment 2, the construction of transgene expression vector
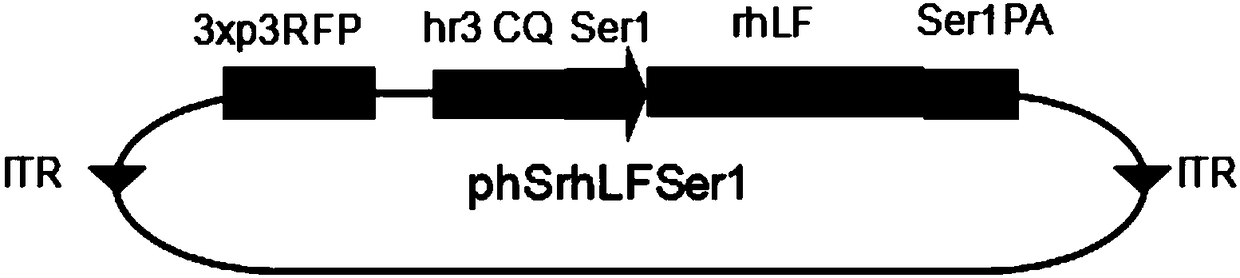
[0033] The commercially synthesized hLF-his6 gene coding sequence was constructed into psl1180[hr3Pser1spRedSer1PA] through BamHI and NotI restriction sites to form psl1180[hr3CQSer1sphLFSer1], the nucleotide sequence is shown in SEQ ID NO.3, and then constructed through the AscI site into the AscI site of the pBac{3xp3EGFPaf} vector to form the transgene expression vector phShLFSer, the vector structure is as follows figure 1 shown.
Embodiment 3
[0034] Embodiment 3, microinjection and fluorescence screening
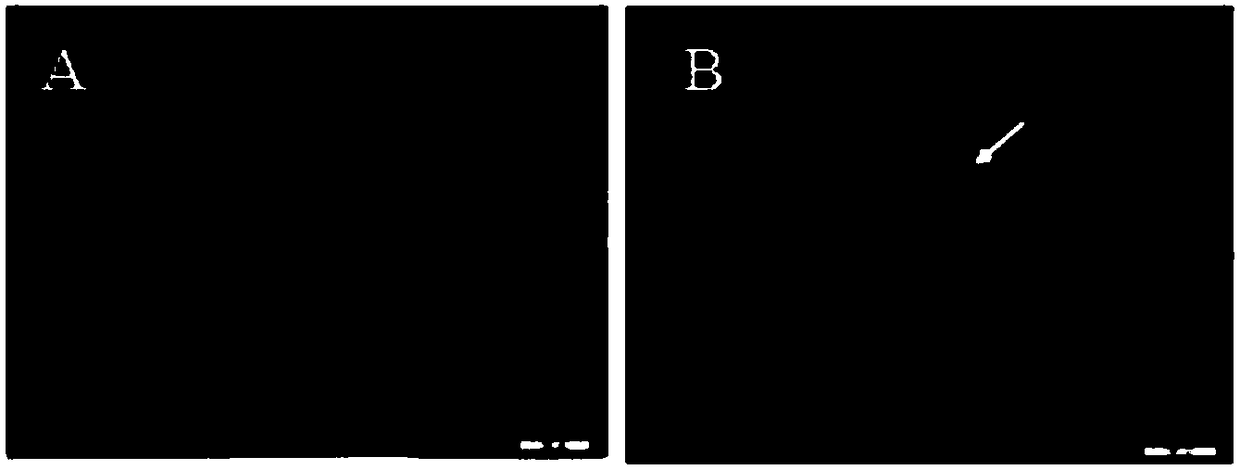
[0035] The transgene expression vector phShLFSer1 and the auxiliary carrier pHA3PIG plasmid were extracted using the QIAGEN Plasimd Mini Kit plasmid extraction kit, and the concentration of each plasmid was diluted to 400 ng / μl, and mixed with the auxiliary carrier pHA3PIG plasmid at a molar ratio of 1:1. Inject the mixed plasmid into Dazao early embryos that have been released from diapause (2-5 hours after oviposition), then seal the injection hole with non-toxic glue, sterilize with 35% formaldehyde steam for 5 minutes, and place at 25°C , hatched in an environment with a relative humidity of 85%, the hatched larvae (G0 generation) were reared with artificial feed, and self-crossed or backcrossed to produce seeds after adulthood, and the obtained G1-generation silkworm eggs (7th day) were exposed to macroscopic stereofluorescence. Detected under a microscope (Olypus MVX10, Japan), red fluorescence was observed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com