Trail receptor-binding agents and uses of same
A technology of use and antibody, applied in the direction of antibody, drug combination, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the limited clinical application value of TNF-α and Fas ligand, toxic and side effects, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
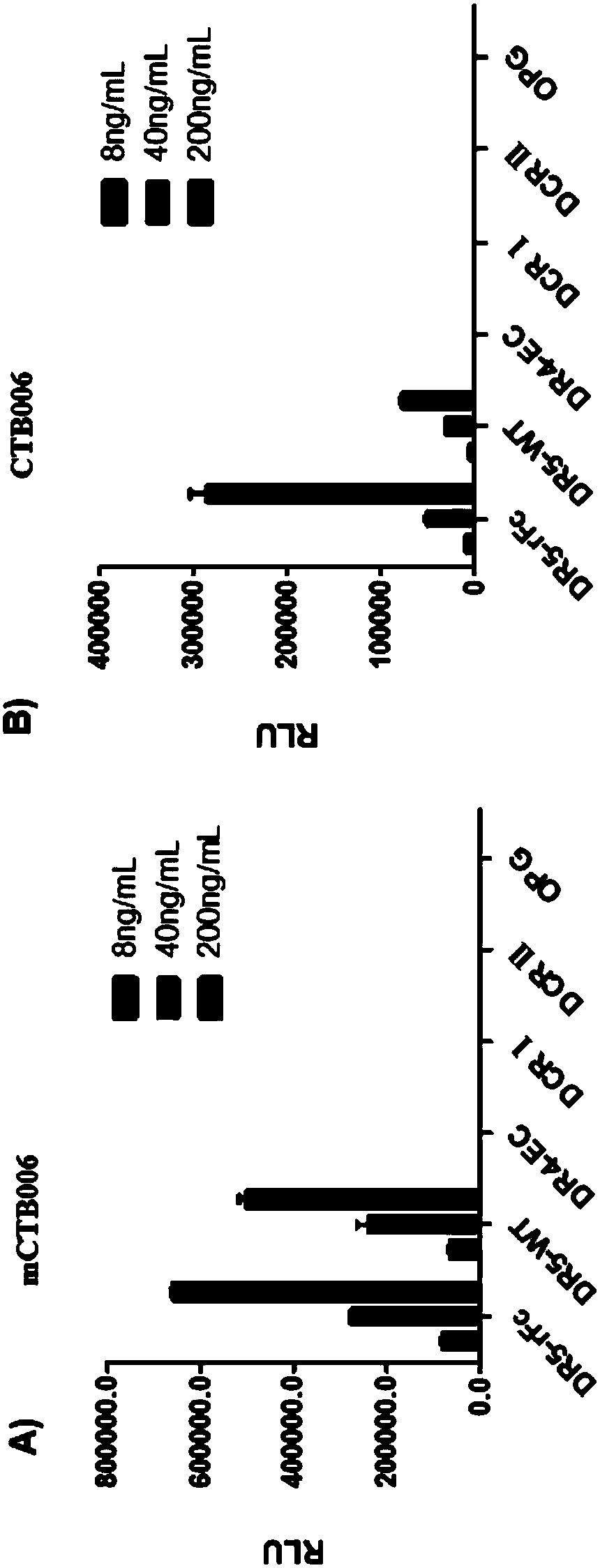
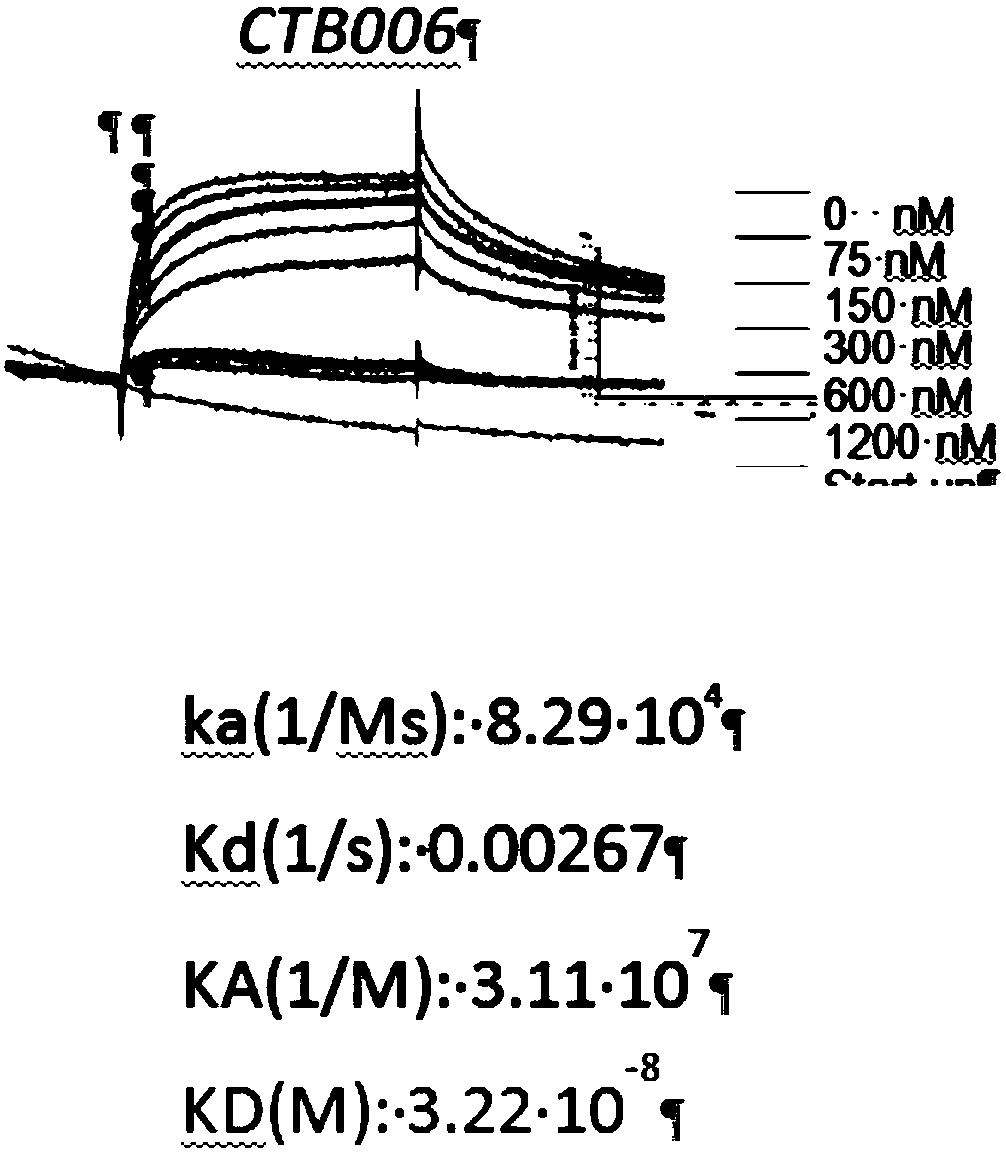
[0327] Characterization of the Binding Specificity and Affinity of Example 1 HUCTB006
[0328] 1.1 Binding specificity
[0329] The binding specificity of HuCTB006 was assessed by chemiluminescent enzyme immunoassay (CLEIA). DR4-His–EC (batch number: 090222-P, 0.72mg / mL, Escherichia coli), DcR1-His (recombinant human TRAIL R3 / TNFRSF10C, Novoprotein Science, batch number: 0328670), DcR2-His (recombinant human TRAIL R4 / TNFRSF10d , Sino Biological, batch number LC06JU1502) and OPG-His (recombinant human osteoprotegerin (recombinant human osteoprotegerin) / TNFRSF11B, Wuxi Pharmatech Co.Ltd, batch number: 20120717) were coated on the microplate, and then it was blocked (Blocked).
[0330] HuCTB006 was diluted to 200, 40 and 8 ng / mL and added to the wells. After incubation for 1 hour at 37°C, the plates were washed. The binding of HuCTB006 to the immobilized antigen was detected with HRP-labeled secondary antibody and substrate solution. The result shows, as figure 1 As shown, t...
Embodiment 2
[0333] In vitro effectiveness of embodiment 2 HuCTB006 treatment
[0334] 2.1 Cytotoxicity of HuCTB006 in human normal tissue cell lines
[0335] Several human normal tissue cell lines, including human lung epithelial fibroblast WI-38, human embryonic lung fibroblast HFL-1, human umbilical vein epithelial cell HUV-EC-C, and human hepatic differentiated cells derived from liver-induced cells (Acquired from Peking University Third Hospital). Cells at 37 °C and 5% CO 2 and culture medium containing 10% FBS. The medium of WI-38 was MEM, and the medium of HFL-1 and HUV-EC-C was F-12K. Cells were treated with oxaliplatin for 24 hours, and then different concentrations of CTB006 were added to the combination group. Results were determined 24 hours after treatment, as image 3 and Figure 4 shown. The examined human normal tissue cells were not sensitive to CTB006, indicating that HuCTB006 had no cytotoxicity to human normal tissue cells.
[0336] 2.2 Cytotoxicity of CTB006 in...
Embodiment 3
[0343] Example 3. In Vivo Efficacy - Human Tumor Cells
[0344] The efficacy of HuCTB006 was tested in a subcutaneous model of nude mice using four human tumor cell lines (ie, two colon, one lung and one pancreatic cancer). The results showed that even at low or micro-dose, HuCTB006 inhibited the tumor growth of colon cancer cells, lung cancer cells and pancreatic cancer cells, while HuCTB006 alone showed the same therapeutic effect as chemotherapy drugs. Results are reproducible.
[0345] 3.1 Efficacy of HuCTB006 in the subcutaneous model of human tumor cells
[0346] 3.1.1 Drugs
[0347] HuCTB006 (batch number H61042F-P01) was obtained from Beijing Cotimes Biotech Co., Ltd.; irinotecan and gemcitabine were obtained from Jiangsu Hengrui Medicine Co., Ltd. ); Paclitaxel was obtained from Beijing Union Pharmaceutical Factory. The drug powder was first dissolved in NS to reach a concentration of 5 mg / ml, and then passed through a 0.2 μm filter membrane (PALL, Syringe Filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com