TRAIL receptor-binding agents and uses of same
A use and antibody technology, applied in the direction of antibodies, applications, drug combinations, etc., can solve the problems of limited clinical application value and toxic side effects of TNF-α and Fas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0177] Preparation of polyclonal antisera and immunogens. Methods of producing antibodies or antibody fragments of the present technology generally involve immunizing a subject (typically a non-human subject such as a mouse or rabbit) with a purified TRAIL receptor polypeptide or with cells expressing a TRAIL receptor polypeptide. Any immunogenic portion of a TRAIL receptor polypeptide can be employed as an immunogen. Suitable immunogenic preparations may contain, for example, recombinantly expressed TRAIL receptor polypeptides or chemically synthesized TRAIL receptor polypeptides. Standard techniques for polyclonal and monoclonal antibody preparation can be used to generate TRAIL receptor binding agents that bind to a TRAIL receptor polypeptide or a portion or fragment thereof using the isolated TRAIL receptor polypeptide or a portion or fragment thereof as an immunogen. Full length TRAIL receptor polypeptides may be used, or alternatively, the present technology provides fo...
Embodiment 1
[0320] Example 1 Characterization of Binding Specificity and Affinity of HUCTB006
[0321] 1.1 Binding specificity
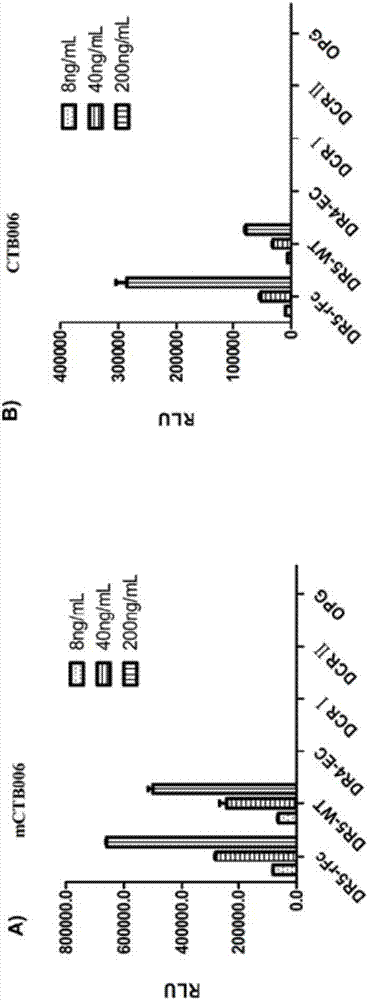
[0322] The binding specificity of HuCTB006 was assessed by chemiluminescent enzyme immunoassay (CLEIA). DR4-His–EC (batch number: 090222-P, 0.72mg / mL, Escherichia coli), DcR1-His (recombinant human TRAIL R3 / TNFRSF10C, Novoprotein Science, batch number: 0328670), DcR2-His (recombinant human TRAIL R4 / TNFRSF10d , Sino Biological, batch number LC06JU1502) and OPG-His (recombinant human osteoprotegerin (recombinant human osteoprotegerin) / TNFRSF11B, Wuxi Pharmatech Co.Ltd, batch number: 20120717) were coated on the microplate, and then it was blocked (Blocked).
[0323] HuCTB006 was diluted to 200, 40 and 8 ng / mL and added to the wells. After incubation for 1 hour at 37, the plates were washed. The binding of HuCTB006 to the immobilized antigen was detected with HRP-labeled secondary antibody and substrate solution. The result shows, as figure 1 As shown, there w...
Embodiment 2
[0326] Example 2 In vitro effectiveness of HuCTB006 treatment
[0327] 2.1 Cytotoxicity of HuCTB006 in human normal tissue cell lines
[0328] Several human normal tissue cell lines, including human lung epithelial fibroblast WI-38, human embryonic lung fibroblast HFL-1, human umbilical vein epithelial cell HUV-EC-C, and human hepatic differentiated cells derived from liver-induced cells (Acquired from Peking University Third Hospital). Cells at 37 °C and 5% CO 2 and culture medium containing 10% FBS. The medium of WI-38 was MEM, and the medium of HFL-1 and HUV-EC-C was F-12K. Cells were treated with oxaliplatin for 24 hours, and then different concentrations of CTB006 were added to the combination group. Results were determined 24 hours after treatment, as image 3 and Figure 4 shown. The examined human normal tissue cells were not sensitive to CTB006, indicating that HuCTB006 had no cytotoxicity to human normal tissue cells.
[0329] 2.2 Cytotoxicity of CTB006 in hu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com