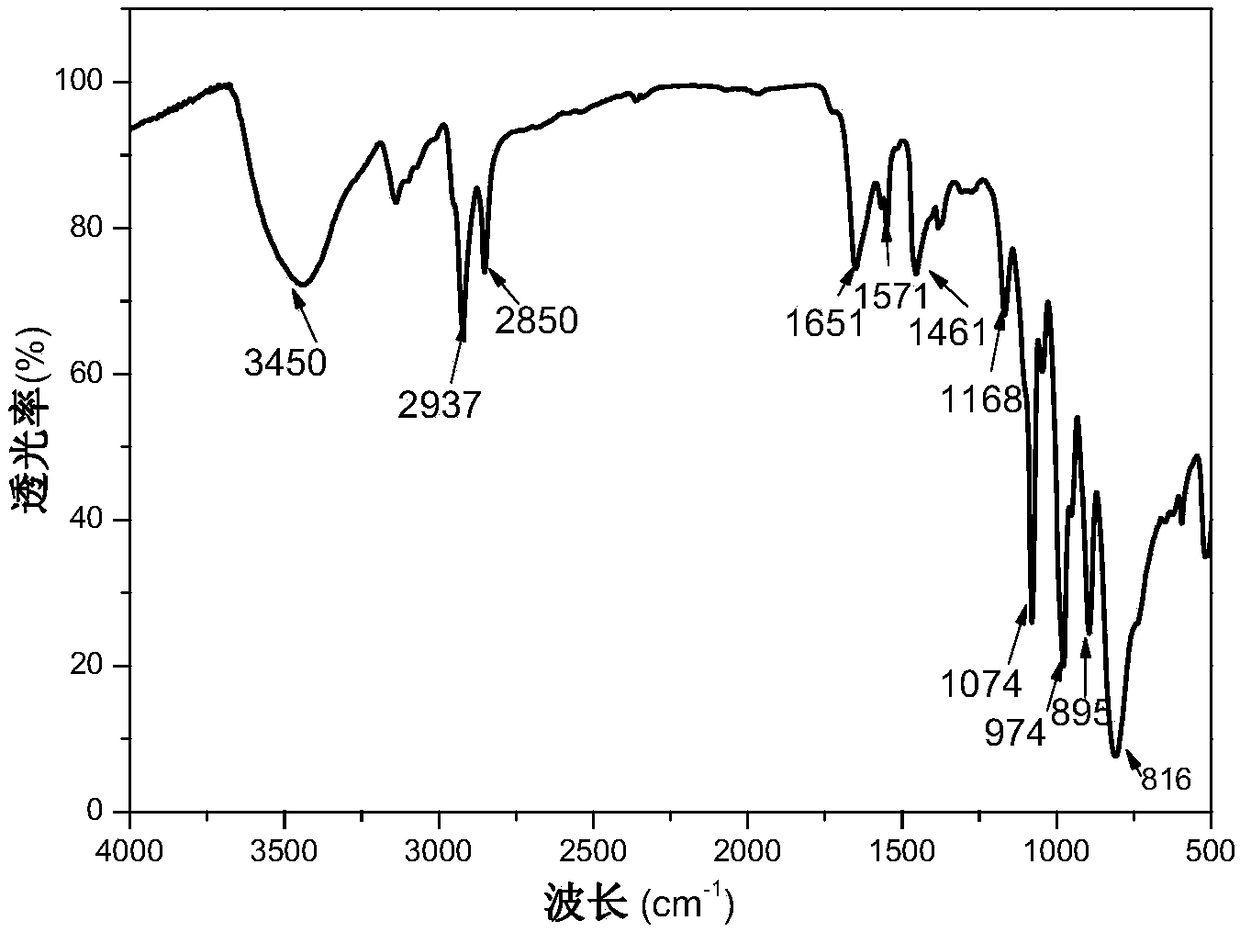
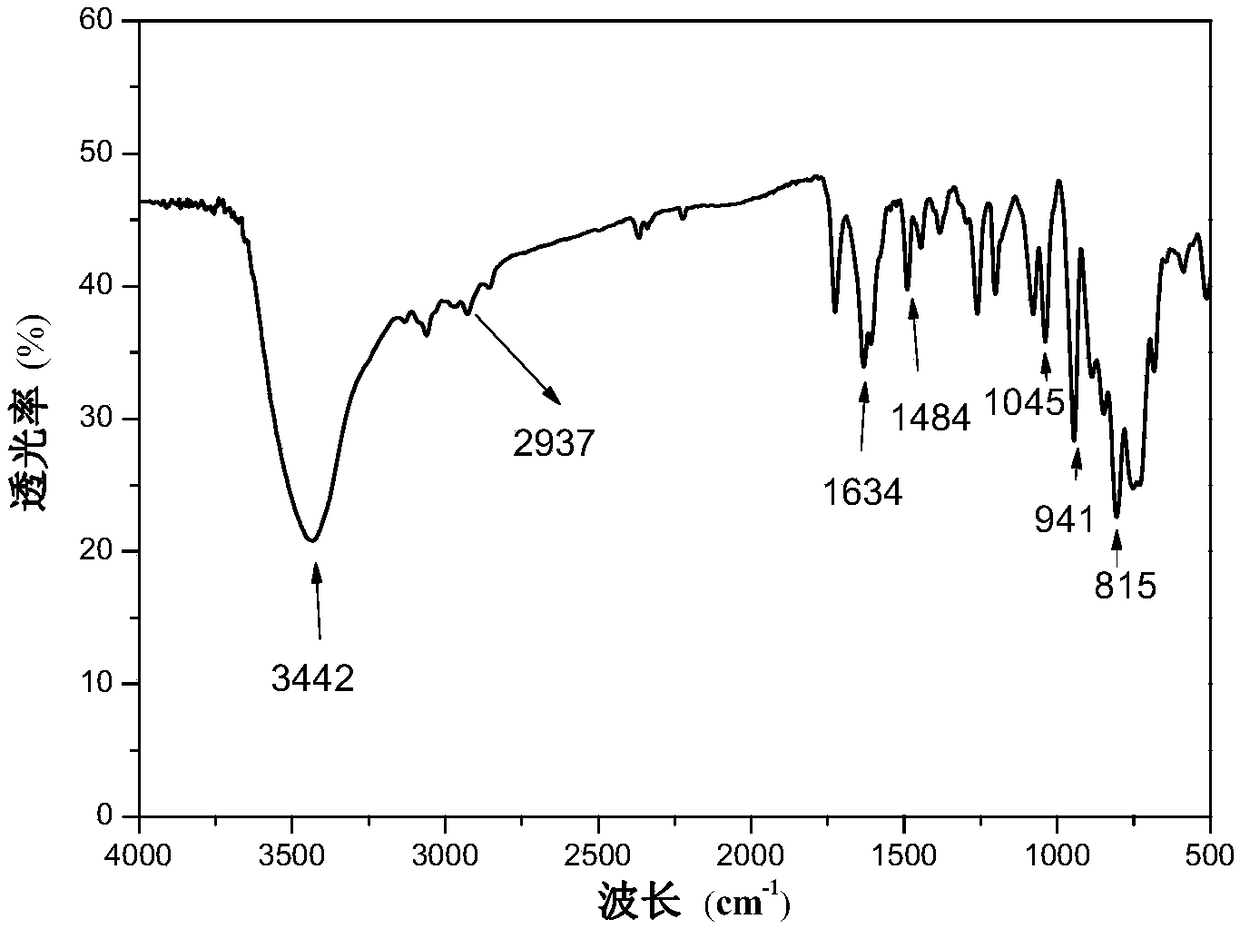
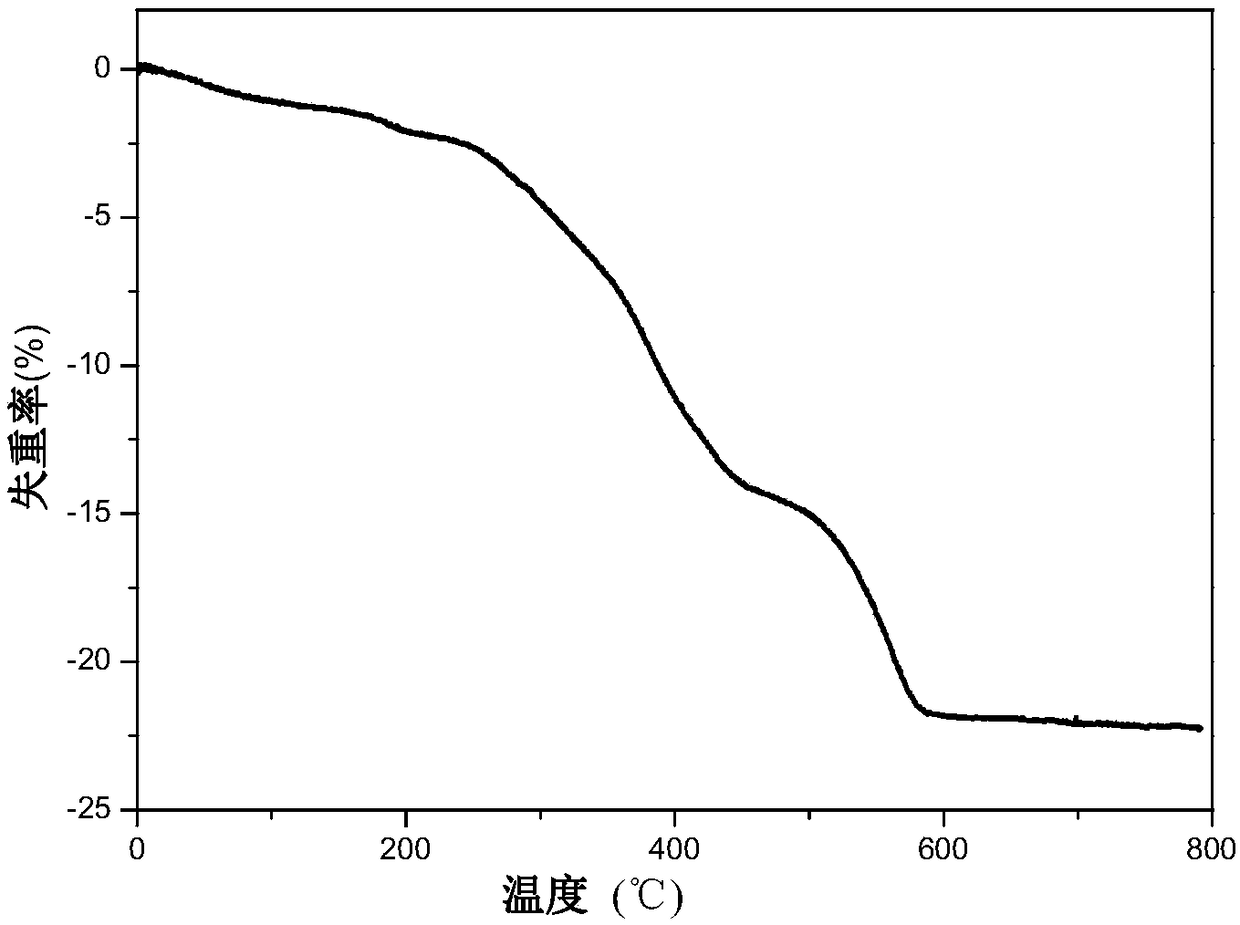
Polyionic liquid-based heteropolyacid catalyst for olefin epoxidation reaction, preparation method and application thereof
A technology of polyionic liquid and epoxidation reaction, which is applied in the direction of physical/chemical process catalyst, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc. It can solve the problems of low utilization rate and difficulty in catalytic recovery, etc. Achieve high utilization rate, mild reaction conditions, and realize the effect of industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Preparation of polyionic liquid-based heteropolyacid catalyst
[0033] (1) Preparation of piperidine functionalized ionic liquid
[0034] Weigh 9.89g of 1-vinylimidazole and 18.40g of N-(2-chloroethyl)piperidine hydrochloride respectively, add them into a 150ml flask, add 40mL of absolute ethanol, mix well, and reflux and stir for 48h under the protection of nitrogen. After the reaction was completed, cool to room temperature, a white solid was precipitated, filtered with suction, washed with dichloromethane for 2-3 times, and dried at 80°C for 2 hours to obtain a white powdery solid particle IMPD.
[0035] (2) Preparation of dodecane functionalized ionic liquid
[0036] Weigh 4.7592g (0.05mol) of 1-vinylimidazole and 12.5529g (0.05mol) of dodecane bromide, mix them into a 100ml round-bottomed flask, add 40mL of absolute ethanol, mix well and reflux under nitrogen protection After 24 hours, the solvent was removed by rotary evaporation to obtain a light yellow liqu...
Embodiment 2
[0044] According to the application of polyionic liquid base heteropolyacid catalyst in embodiment 1, change reactant, concrete method is as follows:
[0045] Weigh 1.08g of 10mmol cyclooctene, 10ml of acetonitrile, and 0.1g of catalyst IMPD-IMDO-PW into a 25ml round bottom flask, put it in a water bath with a temperature controller at 75°C, stir it with a magnetic stirrer, and add it to the reaction system Slowly add 8 mmol of hydrogen peroxide dropwise, and the dropwise reaction ends for 5 hours. After the reaction, the mixture was centrifuged, and the upper layer solution was taken to analyze its composition by gas chromatography, and the lower layer solid could be recovered for the next reaction. The catalyst recovery rate is 86%, the cyclohexene conversion rate is 100%, and the selectivity is 97.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com