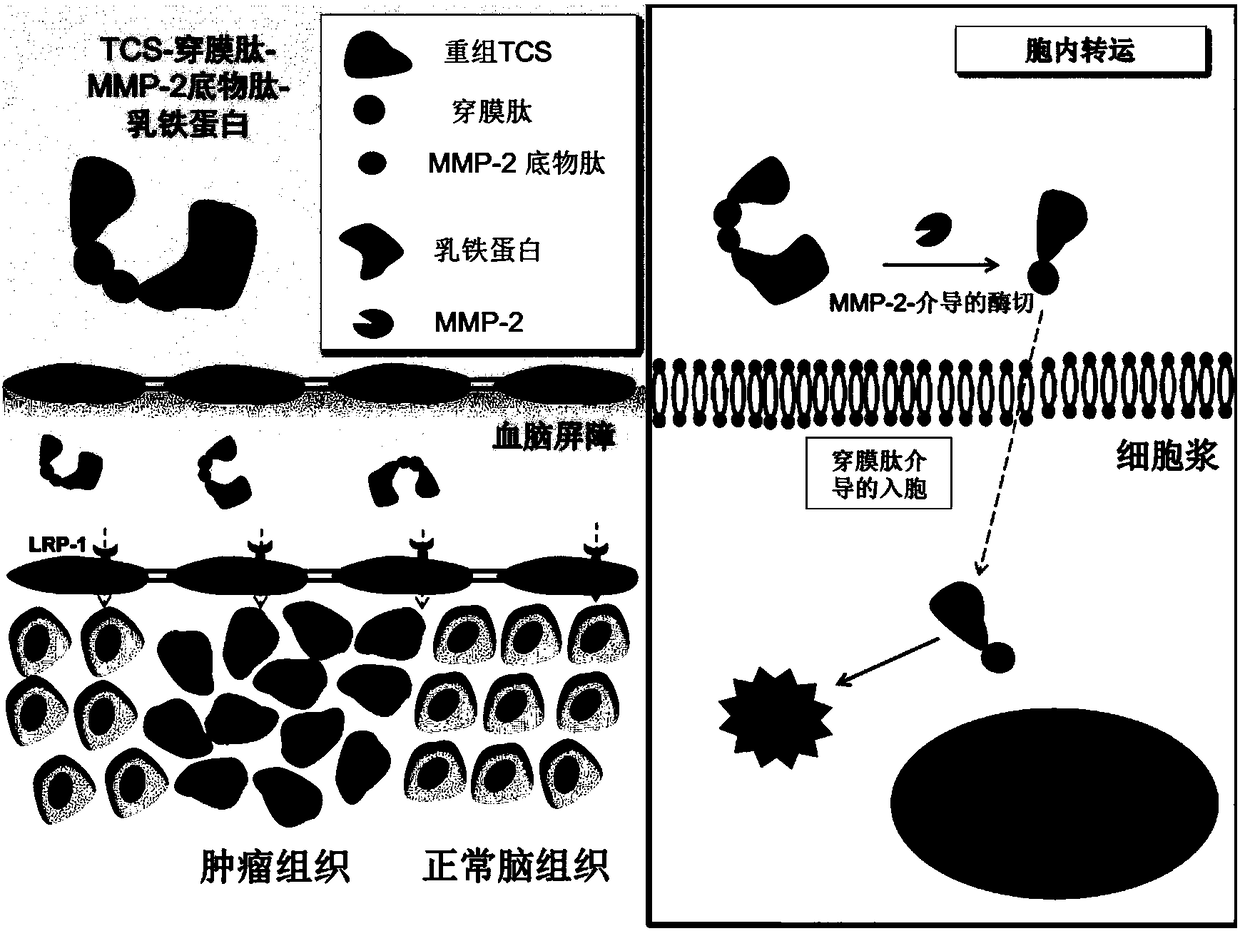
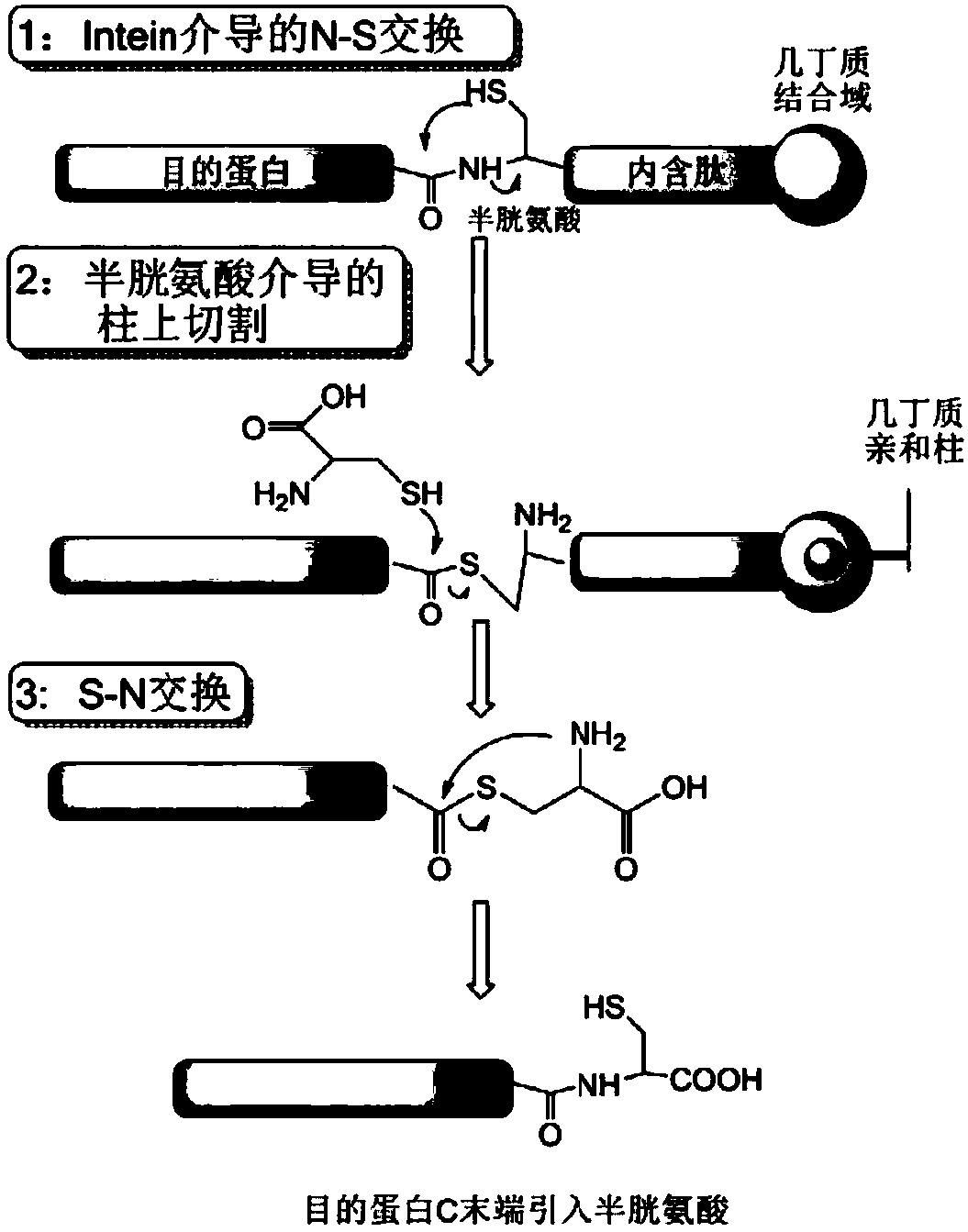
TCS-cell penetrating peptide-tumor protease substrate peptide fusion protein, preparation method and uses thereof
A protease substrate and fusion protein technology, which is applied in the field of TCS-penetrating peptide-tumor protease substrate peptide fusion protein and its preparation, can solve the problems of inhomogeneity of modified products, instability of free sulfhydryl groups, oxidation of sulfhydryl groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0079] Preparation of Recombinant Trichosanthin TCS
[0080] Prokaryotic Expression and Purification of Recombinant Trichosanthin TCS
[0081] a: The TCS recombinant expression plasmid was transformed into Escherichia coli BL21(DE3) competent cells to obtain a strain containing the recombinant plasmid.
[0082] b: Culture the strain containing the recombinant plasmid in LB medium containing 100 μg / ml Amp in a constant temperature shaker at 37°C at 250 rpm until the logarithmic growth phase (absorbance value at 600 nm is 0.6-0.8), and add IPTG with a final concentration of 1 mM , expressed overnight (14h) at 25°C, 150rpm.
[0083] c: Collect the bacterial cells by centrifuging at 6,000 rpm at 4°C for 20 minutes.
[0084] d: Resuspend the bacteria in HEPES buffer (containing 20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5‰ Tween 20, pH 8.5).
[0085] e: Use a probe sonicator with a power of 400 W to sonicate the cells for 30 min.
[0086] f: centrifuge at 12,000 rpm at 4°C for 20 m...
preparation Embodiment 2
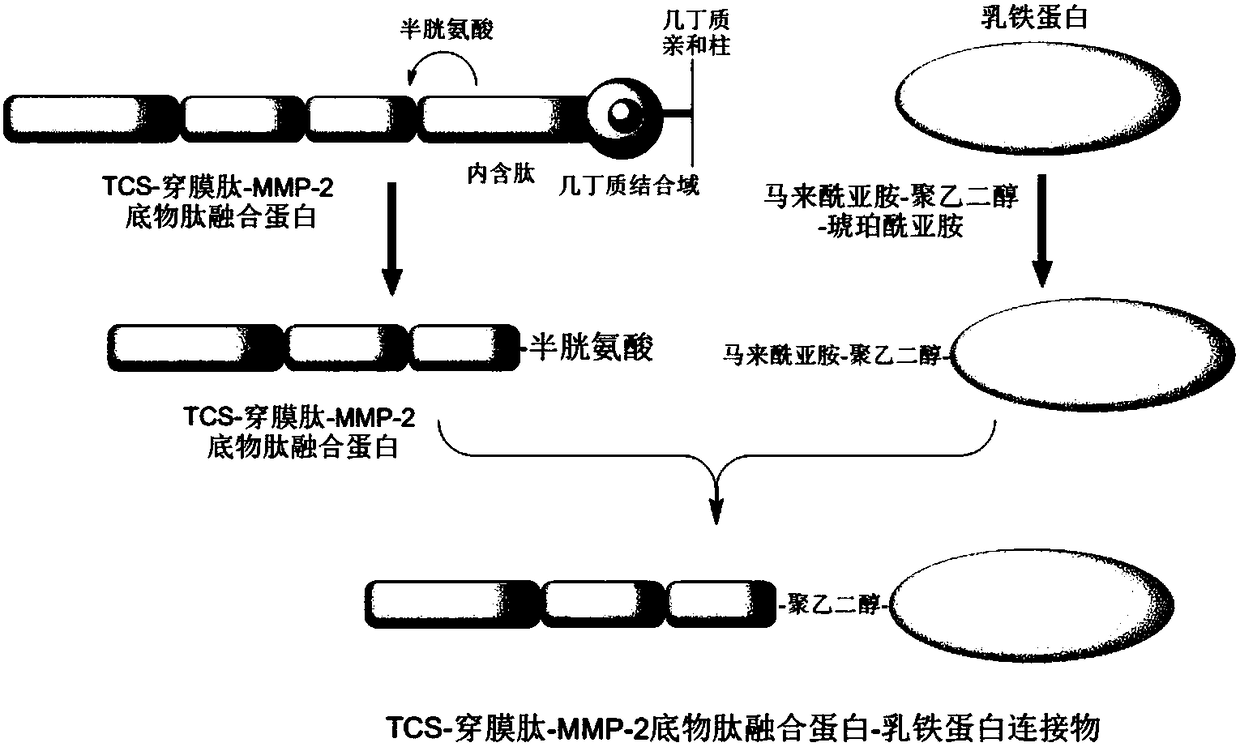
[0092] Synthesis of TCS-penetrating peptide-MMP-2 substrate peptide fusion protein-lactoferrin linker
[0093] (1) Expression and purification of TCS-penetrating peptide-MMP-2 substrate peptide fusion protein
[0094] a: The recombinant expression plasmid of the TCS-penetrating peptide-MMP-2 substrate peptide fusion protein was transformed into Escherichia coli BL21 (DE3) competent cells to obtain a strain containing the recombinant plasmid.
[0095] b: Culture the strain containing the recombinant plasmid in LB medium containing 100 μg / mL Amp in a constant temperature shaker at 37°C at 250 rpm to the logarithmic growth phase (absorbance at 600 nm is 0.6-0.8), and add IPTG at a final concentration of 1 mM , expressed overnight (14h) at 25°C, 150rpm.
[0096] c: Collect the bacterial cells by centrifuging at 6,000 rpm at 4°C for 20 minutes.
[0097] d: Resuspend the bacteria in HEPES buffer (containing 20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5‰ Tween 20, pH 8.5).
[0098] e: ...
experiment Embodiment 1
[0109] Determination of MMP-2 enzyme content in C6 and GL261 cells and their medium
[0110] The MMP-2 enzyme content in C6 and GL261 cells and their culture medium was detected by conventional Western Blot in the field. The specific method is as follows: HT1080 and HUVEC cells in the logarithmic growth phase were digested with trypsin and diluted to 1.5×10 5 The cell suspension of each cell / ml was transferred to a 6-well cell culture plate (Nunc Company), and 2ml of cell suspension was added to each well, and cultured for 4h with DMEM complete medium containing 10% calf serum (37°C, 5% CO 2 ). After the cells adhered to the wall, the medium was discarded, the cells were washed twice with PBS, and replaced with serum-free DMEM medium, 1ml per well, and continued to culture for 24h. The medium supernatant was collected, and the cells were lysed with cell lysate for 30 min, centrifuged at 4°C for 15 min, the supernatant was collected, the protein concentration was determined ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com