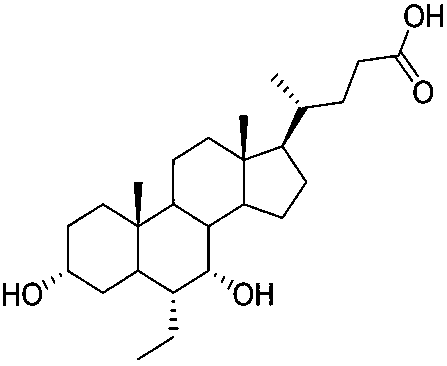
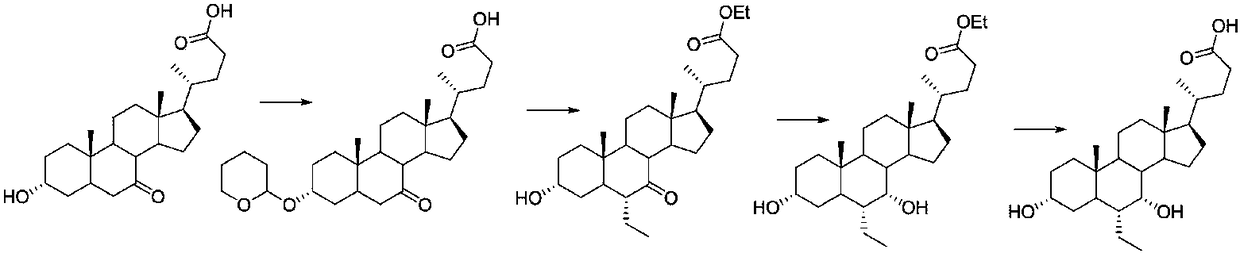
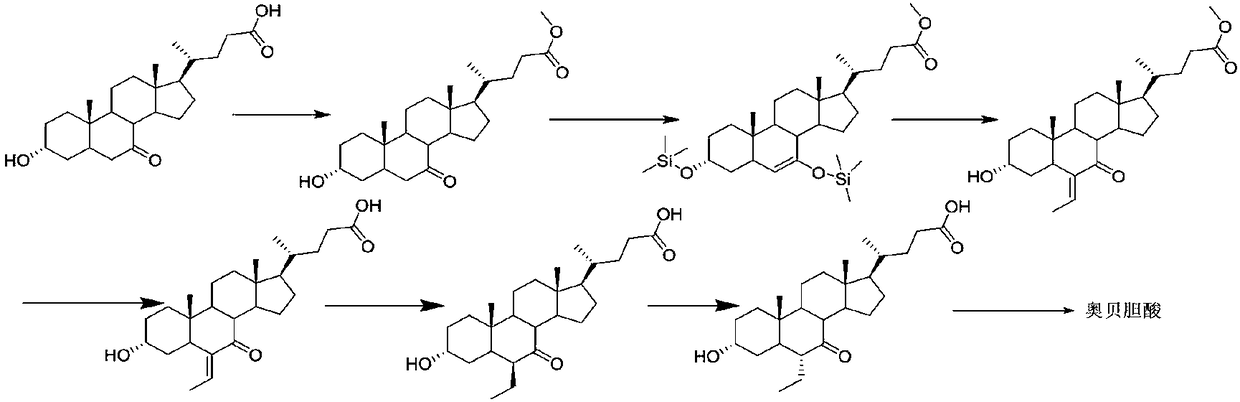
Obeticholic acid derivative and obeticholic acid preparation method
An obeticholic acid and compound technology, which is applied in the field of drug synthesis, can solve the problems of low reaction yield, complicated post-processing, and limited trial range, and achieves the effects of high yield, simple post-processing and simple purification method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: When R 4 and R 5 Form N-methylpiperazinyl for linked rings
[0053] Compound A-2 Preparation Method 1
[0054] Add 50 grams of compound A-1 (1.0eq), 500 milliliters of dichloromethane, nitrogen methyl piperidine (1.2eq), DCC (1.5eq), DMAP (0.2eq), nitrogen protection, room temperature stirring reaction in reaction flask To complete, add water, separate the layers, wash the organic phase with water, dry with anhydrous ammonium sulfate, and concentrate to obtain compound A-2 with a molar yield of 88%. m / z=473.4[M+H] + .
[0055] 1 H NMR (400MHz, Chloroform-d) δ3.55 (ddt, J=15.5, 10.7, 4.6Hz, 1H, C 3 H), 3.36(t, J=7.4Hz, 4H, piperazine hydrogen), 2.20(t, J=7.4Hz, 4H, piperazine hydrogen), 1.30(s, 3H, C 19 on H), 0.66(s,3H, C 18 H above).
[0056] Compound A2 Preparation Method 2
[0057] Add 50 grams of compound A1 (1.0eq), dichloromethane, 0-10°C to the reaction flask, drop in oxalyl chloride, react for 3h, concentrate to dryness, and then add 4-azani...
Embodiment 2
[0064] Embodiment 2 is prepared according to the method of embodiment 1 when R 3 for hydrogen, R 4 Preparation of IV-2 for 4-aminopyridyl
[0065] Add 40 grams of compound A3-2 (1.0eq) to the reaction flask, methylene chloride, nitrogen protection, cool to -60°C to -50°C, add acetaldehyde (2eq), then drop boron trifluoride ether (3eq ), kept at -60°C for 2 hours, raised to room temperature and reacted for 1 hour, added 2N hydrochloric acid, stirred for 30 minutes, filtered, and washed with water to obtain Compound IV-2 with a purity of 93%, Compound A-2 Preparation Method 1, and a molar yield of 90%. m / z=493.3[M+H] + .
Embodiment 3
[0066] Example 3 was prepared according to the method of Example 1. When R3 was hydrogen and R4 was 4-N,N dimethylphenyl, IV-1 was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com