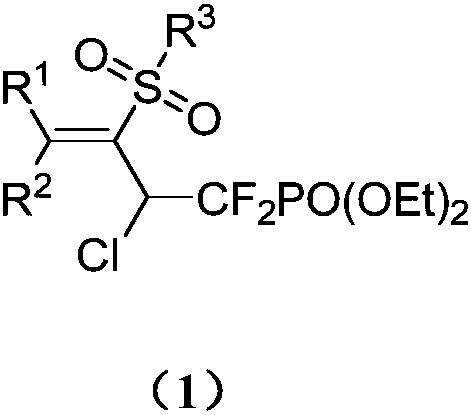
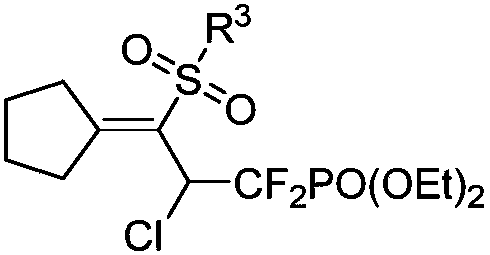
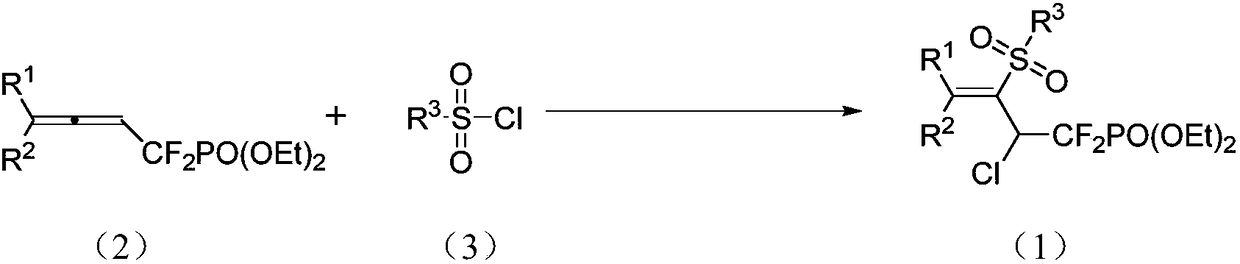
1,1-difluoro-3-sulfonyl-2-chloro-3-butenyl phosphonate compound and synthetic method and application thereof
The technology of a butenyl phosphonate and a synthesis method, which is applied in the field of phosphonate compounds, can solve the problems of complex preparation process, unsatisfactory biological activity, poor regioselectivity, etc., and achieve high regioselectivity, excellent yield, The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Ar or N 2 Under protection, add allene (0.4mmol), benzenesulfonyl chloride (1.2mmol), fac-Ir(ppy) to the reaction tube in sequence 3 (5% mmol), acetonitrile 4mL, normal temperature, reaction under 5W blue light irradiation, TLC monitoring to the end of the reaction, quenching with water, extraction with ethyl acetate, combined organic phases, washed with saturated brine, dried with anhydrous sodium sulfate, filtered and concentrated The product was separated by column chromatography with dichloromethane-ethyl acetate as eluent. reaction
[0030]
[0031] The obtained 1,1-difluoro-3-sulfonyl-2-chloro-3-butenyl phosphonate has the following structure and the yield is 84%.
[0032]
[0033] (2-chloro-1,1-difluoro-4-methyl-3-(phenylsulfonyl)pent-3-en-1-yl)phosphonate IR(neat): 2984,2922,1612,1450,1304,1150,1080, 1033,895,834,756,717,687,602cm -1 ; 1 H-NMR (400MHz, CDCl 3 ): δ7.87-7.85 (m, 2H), 7.58-7.49 (m, 3H), 6.24 (dd, J H-F = 20.2, 11.4 Hz, 1H), 4.35-4.30 (m, 4H), 2.17 (s, ...
Embodiment 2
[0035]
[0036] The structure of the obtained 3-sulfonyl-2-chlorodifluoromethylene allyl phosphonate is as follows, and the yield is 83%.
[0037]
[0038] Diethyl(2-chloro-1,1-difluoro-4-methyl-3-((4-(trifluoromethyl)phenyl)sulfonyl)pent-3-en-1-yl)phosphonate
[0039] IR(neat): 3064, 2983, 2925, 1617, 1453, 1371, 1306, 1151, 1085, 1036, 889, 831, 758, 692, 603cm -1 ; 1 H-NMR (400MHz, CDCl 3 ): δ8.01(d,J=8.0Hz,2H), 7.78(d,J=8.0Hz,2H), 6.19(dd,J H-F = 19.4, 11.8 Hz, 1H), 4.34-4.29 (m, 4H), 2.20 (s, 3H), 2.03 (s, 3H), 1.39 (t, J = 7.0 Hz, 6H); 13 C-NMR (100MHz, CDCl 3 ):δ164.0,146.0,134.6(q,J C-F = 33.1Hz), 130.7, 127.4, 126.2(q, J C-F =3.6Hz),123.0(q,J C-F =271.3Hz),117.3(ddd,J C-F =271.4Hz, J C-F =266.4Hz,J C-P =212.9Hz), 65.2(d, J C-P =6.6Hz),65.1(d,J C-P =6.8Hz),52.7(td,J C-F =J C-P =24.5Hz), 26.1(t, J C-F =5.7Hz),25.1,16.2(d,J C-P =5.0Hz), 16.2(d, J C-P =5.0Hz); 19 F-NMR(376MHz, CDCl 3 ):δ-63.2(s,3F), -105.0to-106.1(m,1F),-107.9to-109.0(m,1F); 31 P-NMR(162MHz, CDCl 3 ...
Embodiment 3
[0041] The operation is the same as before, and the reaction process is as follows:
[0042]
[0043] The structure of the obtained 1,1-difluoro-3-sulfonyl-2-chloro-3-butenyl phosphonate is as follows, and the yield is 70%.
[0044]
[0045] Diethyl(2-chloro-3-cyclopentylidene-1,1-difluoro-3-tosylpropyl)phosphonate IR(neat): 2984,2930,1720,1604,1450,1288,1141,1018,887,810,656cm -1 ; 1 H-NMR (400MHz, CDCl 3 ): δ7.74(d,J=8.4Hz,2H), 7.29(d,J=8.0Hz,2H), 6.09(ddd,J H-F = 20.6, 10.4 Hz, J H-P =1.4Hz, 1H), 4.36-4.26 (m, 4H), 3.04-2.90 (m, 2H), 2.63-2.55 (m, 1H), 2.40 (s, 3H), 2.27-2.18 (m, 1H), 1.76-1.69(m,1H),1.66-1.55(m,2H),1.51-1.44(m,1H),1.38(t,J=7.2Hz,6H); 13 C-NMR (100MHz, CDCl 3 ):δ173.4,144.0,138.4,129.5,127.3,117.5(td,J C-F =271.2Hz,J C-F =266.1Hz,J C-P =212.8Hz), 65.1(d, J C-P =6.6Hz),65.0(d,J C-P =6.9Hz),53.5(td,J C-F = 25.6Hz, J C-P =22.0Hz),36.1(t,J C-P =5.8Hz),35.3,25.9,25.7,21.5,16.3(d,J C-P =5.3Hz), 16.2(d, J C-P =4.4Hz); 19 F-NMR(376MHz, CDCl 3 )δ:-104.2to-105....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com