Preparation method of Vildagliptin intermediate
A technology for intermediates and preparation steps, which is applied in the field of preparation of vildagliptin intermediates, can solve the problems of low yield, unfriendly environment, and a large amount of solvents, and achieve the goals of reducing energy consumption, shortening reaction time, and increasing yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
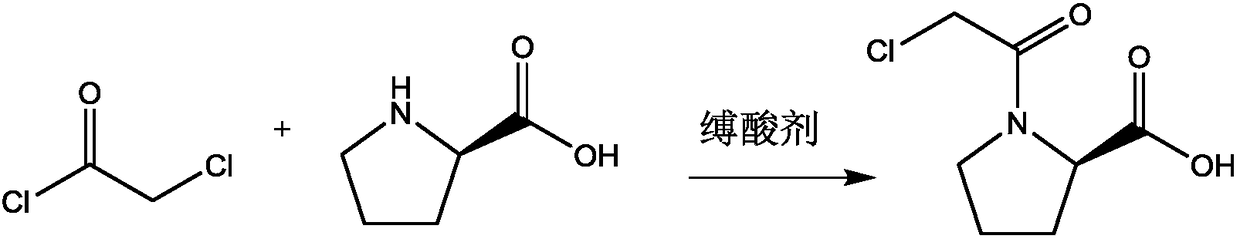
[0019] With a molar ratio of 1:10, weigh L-proline and chloroacetyl chloride, add them into a three-necked flask, and stir and mix at room temperature; after stirring and mixing for 5 minutes, add triethylamine, triethylamine and L- The molar ratio of proline is 2:1, stirred and reacted at room temperature for 3h, recovered excess chloroacetyl chloride by distillation under reduced pressure after the reaction, poured the reaction solution into ethyl acetate and distilled water, stirred and mixed, let stand to separate layers, collected organic layer, and distilled under reduced pressure to recover ethyl acetate to obtain vildagliptin intermediate 1-(2-chloroacetyl)proline, with a melting point of 112.3°C, a purity of 99.89%, and a yield of 92.5%.
example 2
[0021] Weigh L-proline and chloroacetyl chloride at a molar ratio of 1:12, add them to a three-necked flask, and stir and mix at room temperature; after stirring and mixing for 7 minutes, add sodium acetate, sodium acetate and L-proline to the three-necked flask The molar ratio is 2.5:1, stirred and reacted at room temperature for 4h, after the reaction, the excess chloroacetyl chloride was recovered by distillation under reduced pressure, the reaction solution was poured into ethyl acetate and distilled water, stirred and mixed, and allowed to stand for layering, and the organic layer was collected. Ethyl acetate was recovered by pressure distillation to obtain vildagliptin intermediate 1-(2-chloroacetyl)proline, with a melting point of 112.7°C, a purity of 99.92%, and a yield of 93.0%.
example 3
[0023] With a molar ratio of 1:15, weigh L-proline and chloroacetyl chloride, add them to a three-necked flask, and stir and mix at room temperature; after stirring and mixing for 10 minutes, add 4-dimethylaminopyridine, 4-dimethylaminopyridine, and 4-dimethylaminopyridine to the three-necked flask The molar ratio of aminopyridine and L-proline is 3:1. Stir the reaction at room temperature for 5 hours. After the reaction, distill under reduced pressure to recover excess chloroacetyl chloride. Pour the reaction solution into ethyl acetate and distilled water, stir and mix, and then let stand Separate layers, collect the organic layer, and recover ethyl acetate by distillation under reduced pressure to obtain vildagliptin intermediate 1-(2-chloroacetyl)proline, with a melting point of 113.1° C., a purity of 99.92%, and a yield of 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com