Primer and probe for identifying dual real-time fluorescence quantitative PCR (polymerase chain reaction) of A type and B type cattle pasteurella multocida and detection method
A technology for real-time fluorescence quantification and Pasteurella, which is applied in the direction of microbial-based methods, biochemical equipment and methods, and microbial determination/inspection, can solve the problem of not identifying Type A and Type B Pasteurella multocida, Weak cross-protection of different serotypes to achieve the effect of ensuring safety and rationality, high sensitivity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1. is designed to be used for the primer of the dual real-time fluorescent quantitative PCR of identification A type and B type Bovine Pasteurella multocida Objects and TaqMan Probes
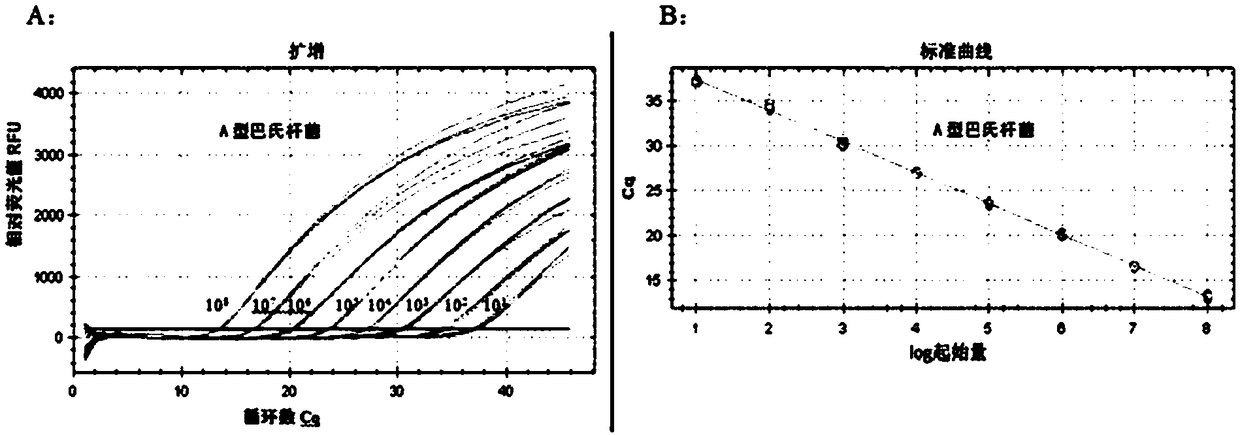
[0063] Since the nucleotide sequences of Type A and Type B bovine Pasteurella multocida are relatively large, it is key to find better different site specificity for the detection of Type A and Type B bovine Pasteurella multocida. Retrieve from NCBI's nucleic acid database GenBank (http: / / www.ncbi.nlm.nih.gov) to obtain the regional genes of type A bovine Pasteurella multocida hyaD-hyaC (Pasteurella multocida capsule biosynthesis gene cluster, GenBank number: AF067175.2) and the sequence of the region gene of Pasteurella multocida capsulebiosynthesis gene cluster, complete sequence GenBank: AF169324) of Type B bovine Pasteurella multocida bcbD were compared with DNAMan software, and designed according to primers and TaqMan probes In principle, select the regional gene of Pa...
Embodiment 2
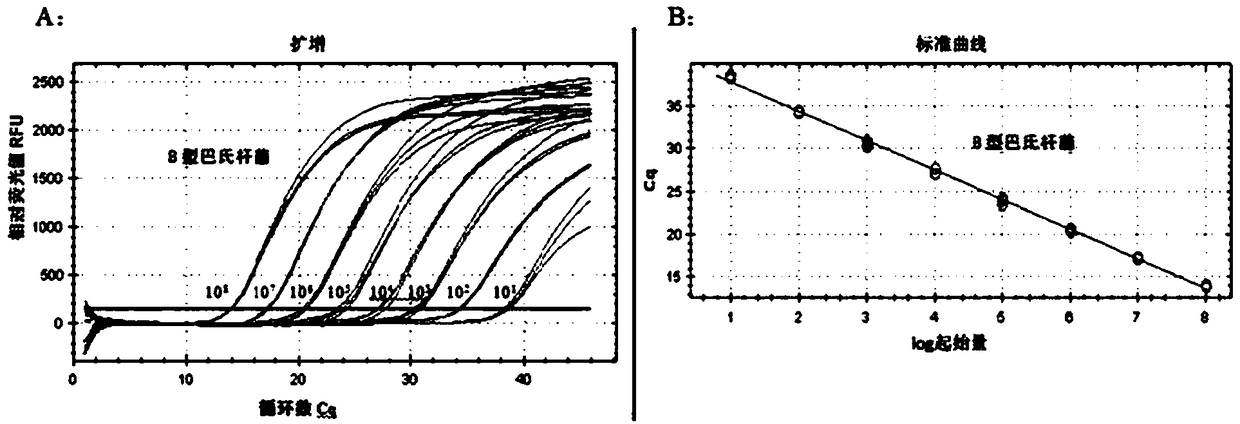
[0077] Embodiment 2. Use primers of the present invention and TaqMan probe to carry out double-acting to type A and type B bovine Pasteurella multocida Heavy real-time fluorescent quantitative PCR differential detection
[0078] Step 1. Extract genomic DNA from the sample
[0079] Genomic DNA is extracted from the bacterial cultures of Type A and Type B bovine Pasteurella multocida (for obtaining standard items and obtaining positive controls) and samples to be tested, and the specific method includes the following steps ( Blood & Tissue Kit, QUAIGEN Company):
[0080] (1) QUAIGEN kit preparation: According to the kit instructions, prepare (96%-100%) ethanol in advance and add absolute ethanol of specified concentration to reagent Buffer AW1 and Buffer AW2.
[0081] (2) Add 100 μL of sample to a 1.5 mL centrifuge tube, add 20 μL proteinase K and 180 μl BufferATL, and vortex to mix.
[0082] (3) In the 1.5mL centrifuge tube in step (2), add 200μL Buffer AL to the mixtur...
Embodiment 3
[0112] Embodiment 3. Specificity experiment
[0113] Utilize QUAIGEN kit according to the method for embodiment 2 to A type, B type, D type, E type and F type Pasteurella multocida, Brucella, bovine type A clostridium, bovine type B clostridium, Bovine Clostridium type C, bovine Clostridium type D, porcine circovirus type 2 (PCV2), rhinotracheitis virus (IBRV) extract DNA, against foot-and-mouth disease type A, foot-and-mouth disease type O, foot-and-mouth disease Asia type, bovine viral diarrhea virus (BVDV), bovine epidemic fever virus extract RNA, simultaneously take the DNA that type A and type B bovine Pasteurella multocida bacterial culture extracts as a positive control, and use non-enzyme water as a negative control, primers of the present invention and TaqMan probe Under the guidance of the needle, double real-time fluorescent quantitative PCR detection was performed, and the PCR reaction system and reaction conditions were referred to in Example 2 to verify the spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com