Fluorine modified Fe3O4 magnetic nano material and preparation method and applications thereof
A magnetic nanometer and fluorine modification technology, applied in chemical instruments and methods, water/sludge/sewage treatment, water pollutants, etc., can solve problems that are difficult to meet practical applications, difficult to adsorb and degrade, and long reaction time. Achieve the effects of easy magnetic separation and recovery, easy operation and control, and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of fluorine modified Fe 3 o 4 Magnetic nanomaterial, its preparation method comprises the steps: weighing 1.856g FeCl 3 ·6H 2 O was dissolved in 4.5 mL ethylene glycol at 50 °C, and 1.801 g CH 3 COONa was stirred for 10 min, then 0.3 g of NaF was added and stirred for 10 min to obtain a reaction liquid; the resulting reaction liquid was transferred to a reaction kettle, reacted at 198°C for 14 h, then quenched to room temperature, and the obtained black solid was collected by magnetic separation and washed once with ethanol , washed four times with deionized water, and dried at 60°C for 12 hours to obtain the final product.
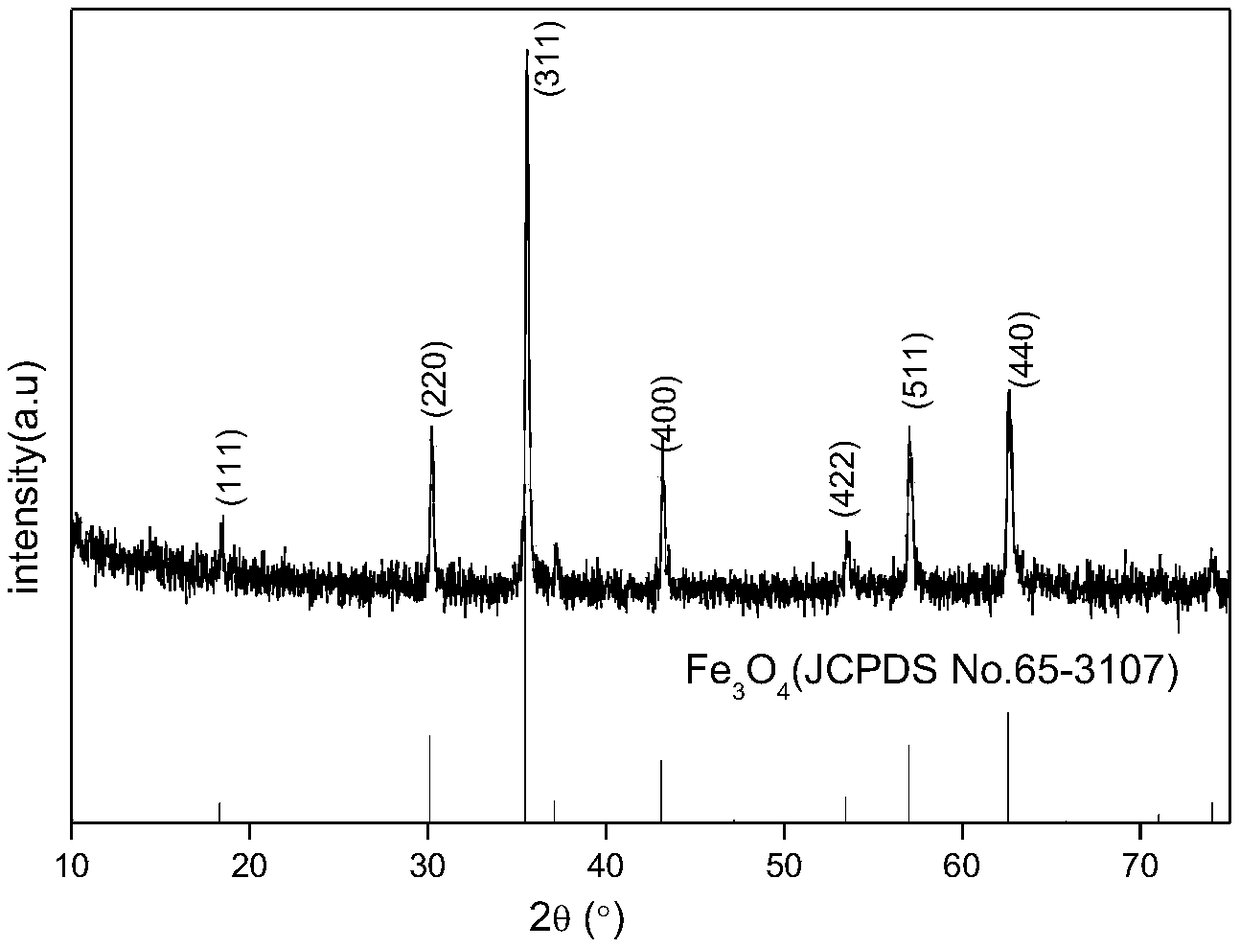
[0030] The XRD spectrum ( figure 1 ) and Fe 3 o 4 The standard collection of illustrative plates is consistent (JCPDS No.65-3107), shows that product particle has cubic inverse spinel structure; The F1s XPS spectrum of gained product ( figure 2 ) The two peaks at 684eV and 686eV indicate that fluorine exists in two ways: surface fluo...
Embodiment 2
[0032] A kind of fluorine modified Fe 3 o 4 Magnetic nanomaterial, its preparation method comprises the steps: weighing 1.856g FeCl 3 ·6H 2 O was dissolved in 4.5 mL ethylene glycol at 50 °C, and 1.801 g CH 3COONa was stirred for 10 min, and then 0.15 g of NaF was added to continue stirring for 10 min to obtain a reaction solution; the resulting reaction solution was transferred to a reaction kettle, and reacted at 198 ° C for 14 h, then quenched to room temperature, and the obtained black solid was collected by magnetic separation and washed once with ethanol , washed four times with deionized water, and dried at 60°C for 12 hours to obtain the final product.
Embodiment 3
[0034] A kind of fluorine modified Fe 3 o 4 Magnetic nanomaterial, its preparation method comprises the steps: weighing 1.856g FeCl 3 ·6H 2 O was dissolved in 4.5 mL ethylene glycol at 50 °C, and 1.801 g CH 3 COONa was stirred for 10 min, then 0.03 g of NaF was added and stirred for 10 min to obtain a reaction liquid; the resulting reaction liquid was transferred to a reaction kettle, reacted at 198 °C for 14 h, then quenched to room temperature, and the obtained black solid was collected by magnetic separation and washed once with ethanol , washed four times with deionized water, and dried at 60°C for 12 hours to obtain the final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com