Triamcinolone acetonide soluble micro-needle and preparation method thereof
A soluble, microneedle technology, applied in microneedles, needles, pharmaceutical formulations, etc., can solve the problems of insufficient skin puncture efficiency, low solubility of triamcinolone acetonide, and reduced drug delivery efficacy, so as to reduce the difficult to control and improve the dosage of drug delivery. Needle tip drug loading rate and risk reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of triamcinolone acetonide soluble microneedles
[0048] In this embodiment, triamcinolone acetonide soluble microneedles (referred to as TA-DMNA-1) are prepared. The shape of the needle tip is bullet-shaped, the height of the cylindrical support structure of the needle tip is 700 μm, and the height of the conical needle tip structure is 500 μm. The diameter of the bottom of the cone is 300 μm, the distance between the tops of the cones of two adjacent needle points is 900 μm, and the base is a circle with a diameter of 2 cm. The preparation of the soluble microneedle comprises the following steps:
[0049] 1. Preparation of drug inclusion complex solution (TA-HP-β-CD-IC)
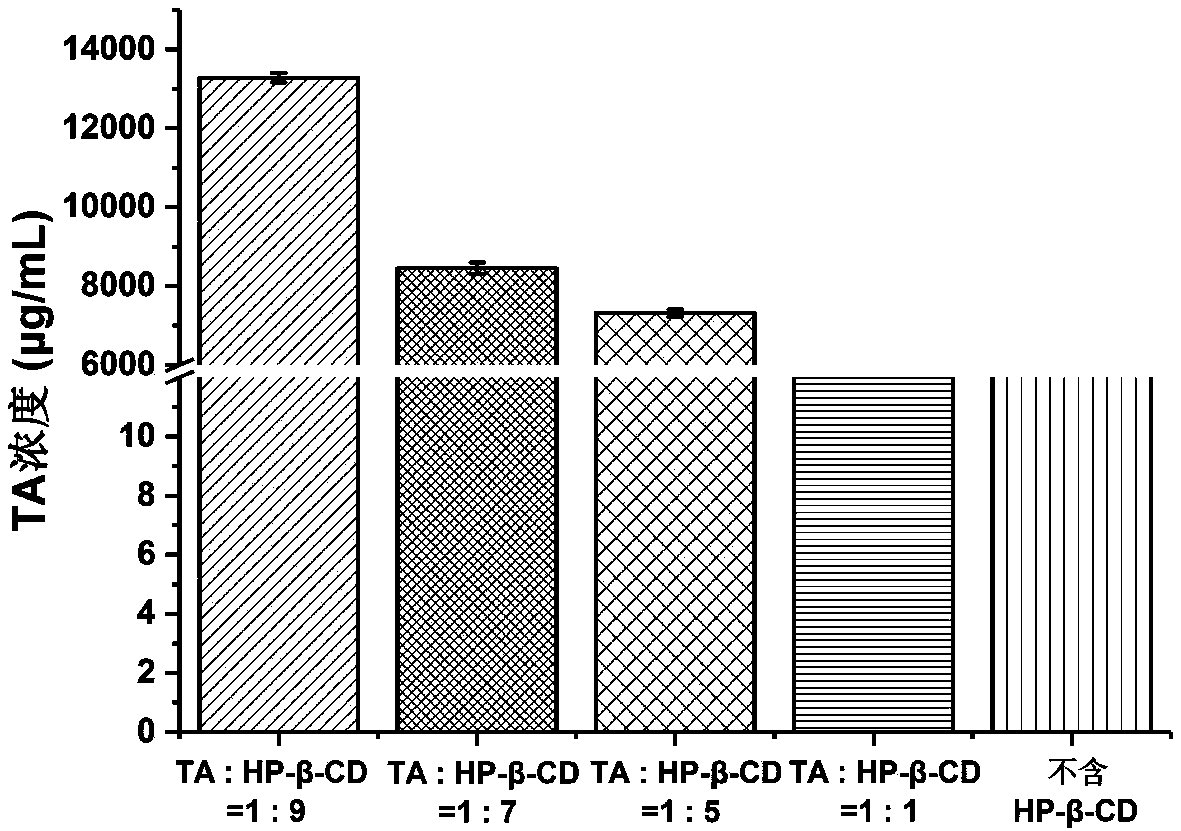
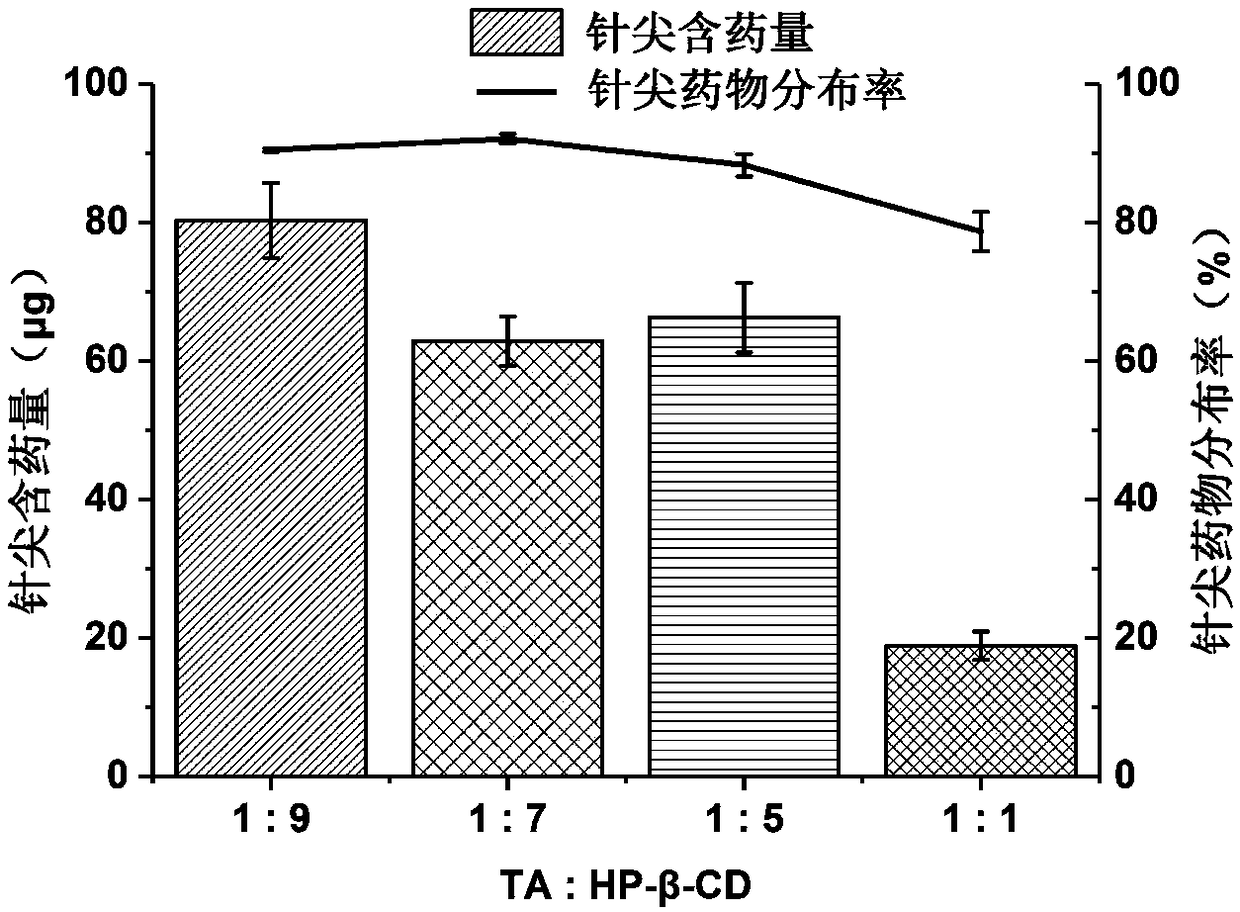
[0050] Accurately weigh 0.4345 g (0.001 mol) of triamcinolone acetonide (TA) and 12.8427 (0.009 mol) of hydroxypropyl-β-cyclodextrin (HP-β-CD), add 10 mL of water, stir to dissolve, and use magnetic stirring Stir at 200rpm for 4 hours, centrifuge at 4000rpm for 10min to take t...
Embodiment 2
[0060] Example 2 Preparation of triamcinolone acetonide soluble microneedles
[0061] In this example, the preparation of triamcinolone acetonide soluble microneedles (TA-DMNA-2) is carried out. The shape of the needle tip is bullet-shaped, the height of the cylindrical support structure of the needle tip is 700 μm, and the height of the conical needle tip structure is 500 μm. The diameter of the bottom is 300 μm, the distance between the tops of the cones of two adjacent needle tips is 900 μm, and the base is a circle with a diameter of 2 cm. The preparation of the soluble microneedle comprises the following steps:
[0062] 1. Preparation of TA-HP-β-CD-IC aqueous solution
[0063] Accurately weigh 0.4345 g (0.001 mol) of triamcinolone acetonide raw material, 12.8427 g (0.009 mol) of HP-β-CD, add water 10 mL, stir to dissolve, and use a magnetic stirrer to stir at 200 rpm for 4 hours, and centrifuge at 4000 rpm for 10 min Take the supernatant to obtain the TA-HP-β-CD-IC aque...
Embodiment 3
[0073] Example 3 Preparation of triamcinolone acetonide soluble microneedles
[0074] In this example, the preparation of triamcinolone acetonide soluble microneedles (TA-DMNA-3) is carried out. The shape of the needle tip is bullet-shaped, the height of the cylindrical support structure of the needle tip is 700 μm, and the height of the conical needle tip structure is 500 μm. The diameter of the bottom is 300 μm, the distance between the tops of the cones of two adjacent needle tips is 900 μm, and the base is a circle with a diameter of 2 cm. The preparation of the soluble microneedle comprises the following steps:
[0075] 1. Preparation of TA-HP-β-CD-IC aqueous solution
[0076]Accurately weigh 0.4345g (0.001mol) of triamcinolone acetonide raw material, 12.8427g (0.009mol) of HP-β-CD, add 10mL of water, stir to dissolve, and use a magnetic stirrer to stir at a speed of 200rpm for 4 hours, and centrifuge at 4000rpm for 10min Take the supernatant to obtain the TA-HP-β-CD-IC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com