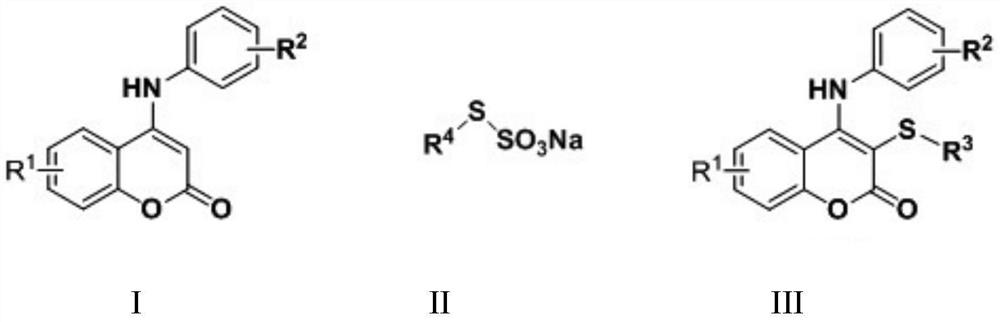
A kind of synthetic method of thiocoumarin
A technology of thiocoumarin and synthesis method, which is applied in the field of synthesis of thiocoumarin, can solve the problems of unavailable raw materials, peculiar smell, high cost, etc., and achieve the effect of convenient separation and purification, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
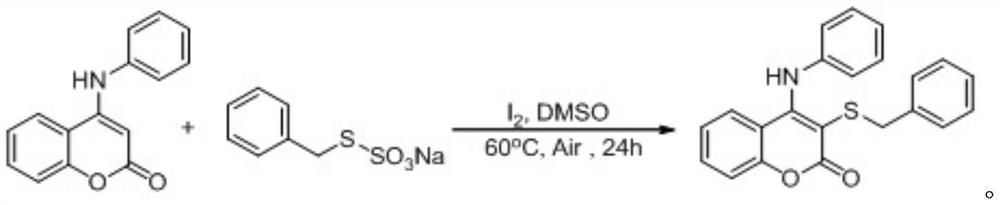
[0030] At room temperature, add simple iodine (0.04 mmol), aminocoumarin (0.2 mmol), S-benzyl thiosulfate O-sodium into a 25 ml Schlenk tube equipped with a magnetic stirrer Salt (0.3 mmol), 2 mL DMSO was added under air, and the reaction tube was placed in an oil bath at 60° C. and stirred for 24 h. After the reaction was completed, the resulting solution was cooled to room temperature, 5 ml of water and 20 ml of ethyl acetate were added to the reaction solution for extraction 4 times, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specifications: 200 mesh to 300 mesh, eluent petroleum ether / ethyl acetate (10:1, v / v)) to obtain 53 mg of the corresponding target product with a yield of 74% .
[0031] The chemical reaction formula is:
[0032]
[0033] The obtained product nuclear magnetic spectrum data are:
[0034] 1 H NMR (CDCl 3 ,500MHz,ppm)δ7.72(s,1H),7.40-7.37(m,1H),7.30(d,1H...
Embodiment 2
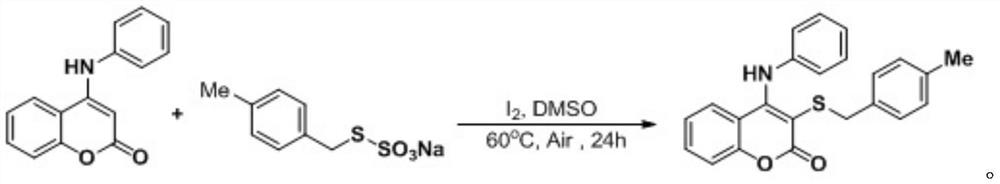
[0039] At room temperature, add simple iodine (0.04 mmol), aminocoumarin (0.2 mmol), S-4-methylbenzyl thiosulfate into a 25 ml Schlenk tube equipped with a magnetic stirrer Ester O-sodium salt (0.3 mmol), 2 mL DMSO was added under air, and the reaction tube was placed in an oil bath at 60°C and stirred for 24 h. After the reaction was completed, the resulting solution was cooled to room temperature, 5 ml of water and 20 ml of ethyl acetate were added to the reaction solution for extraction 4 times, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specifications: 200 mesh to 300 mesh, eluent petroleum ether / ethyl acetate (10:1, v / v)) to obtain 67 mg of the corresponding target product with a yield of 90% .
[0040] The chemical reaction formula is:
[0041]
[0042] The obtained product nuclear magnetic spectrum data are:
[0043] 1 H NMR (CDCl 3 ,500MHz,ppm)δ7.84(s,1H),7.40(s,1H),7....
Embodiment 3
[0047] At room temperature, add simple iodine (0.04 mmol), aminocoumarin (0.2 mmol), S-phenylethyl thiosulfate O- Sodium salt (0.3 mmol), 2 mL DMSO was added under air, and the reaction tube was placed in an oil bath at 60° C. and stirred for 24 h. After the reaction was completed, the resulting solution was cooled to room temperature, 5 ml of water and 20 ml of ethyl acetate were added to the reaction solution for extraction 4 times, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specifications: 200 mesh to 300 mesh, eluent petroleum ether / ethyl acetate (10:1, v / v)) to obtain 62 mg of the corresponding target product with a yield of 83% .
[0048] The chemical reaction formula is:
[0049]
[0050] The obtained product nuclear magnetic spectrum data are:
[0051] 1 H NMR (CDCl 3 ,500MHz,ppm)δ7.74(s,1H),7.45-7.42(m,1H),7.33(t,3H,J
[0052] =15.0Hz), 7.21-7.14(m, 7H), 7.01(d, 2H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com