Finasteride sustained release tablets and preparation method thereof
A technology for finasteride and sustained-release tablets, applied in the field of finasteride sustained-release tablets and its preparation, can solve the problems of low drug bioavailability, slow disintegration speed, no sustained-release effect, etc., and achieve prolongation The effect of drug action time, promoting disintegration speed, and improving drug bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
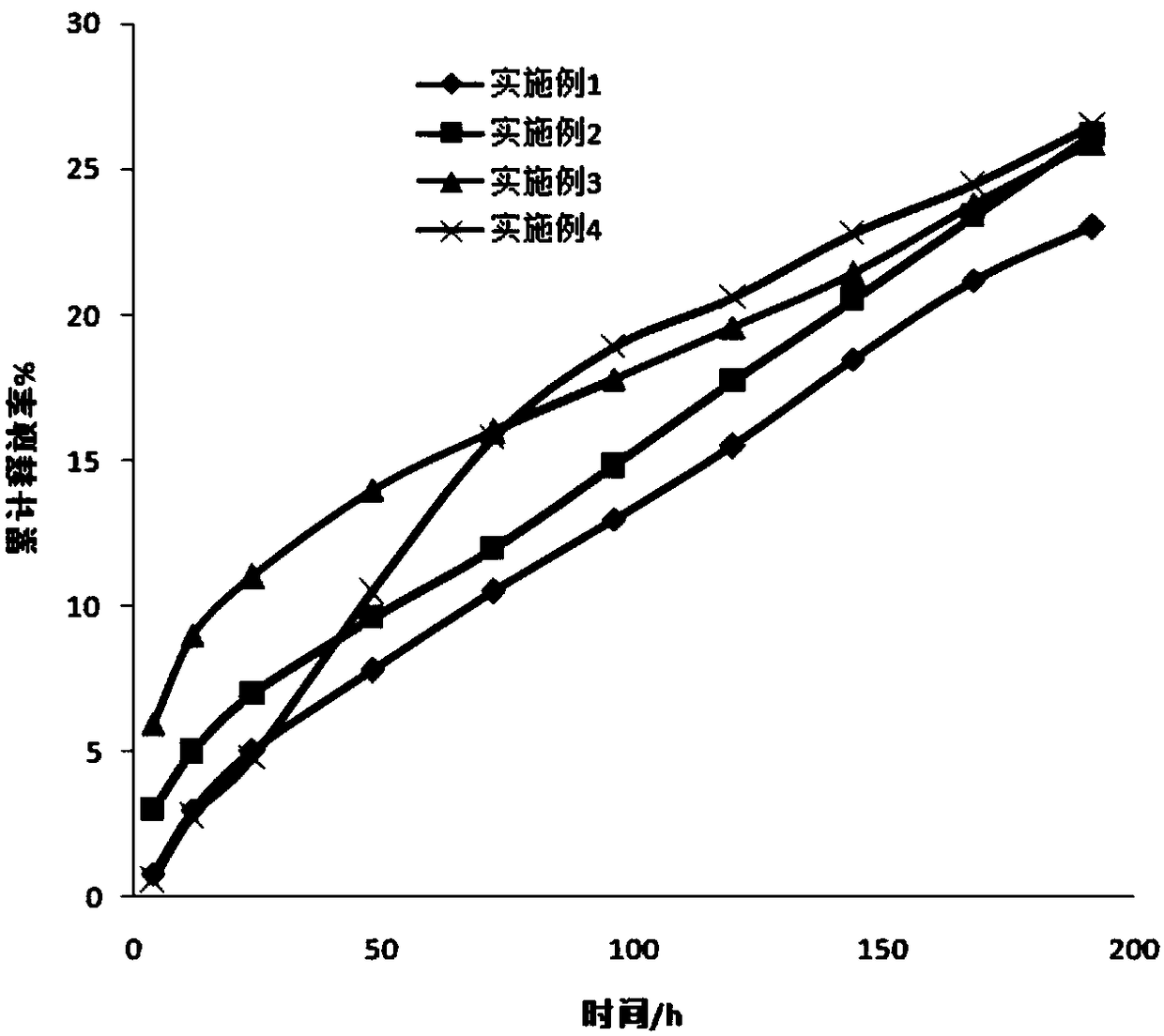
[0032] In this embodiment, a finasteride sustained-release tablet is prepared from the following raw materials: phosphatidylcholine 120g, glyceryl dioleate 60g, ethanol 55g, finasteride 4g, micropowder silica gel 30g, chitosan 40g , 40 g of polyvinylpyrrolidone, 6 g of low-substituted hydroxypropyl cellulose, 2 g of magnesium stearate, and 0.5 g of mannitol. According to the method above, 1000 sustained-release tablets of this example were prepared, each containing about 4 mg of finasteride.
[0033] All the tablets prepared in this embodiment are easy to demould, have a uniform and smooth surface, and have no cracking or loosening. Take six tablets and test their average disintegration speed in water is 25s.
Embodiment 2
[0035] A finasteride sustained-release tablet in this embodiment is prepared from the following raw materials: phosphatidylethanolamine 120g, glyceryl trioleate 60g, ethanol 55g, finasteride 4g, micropowder silica gel 30g, chitosan 40g, Carboxymethylcellulose sodium 40g, low-substituted hydroxypropylcellulose 6g, zinc stearate 2g, sorbitol 0.5g. According to the method above, 1000 sustained-release tablets of this example were prepared, each containing about 4 mg of finasteride.
[0036] The tablets prepared in this embodiment are easy to demould, and 99.99% of the tablets have a uniform and smooth surface without cracking or loosening. Take six tablets and test their average disintegration speed in water is 26s.
Embodiment 3
[0038] A finasteride sustained-release tablet in this embodiment is prepared from the following raw materials: phosphatidylserine 100g, glyceryl stearate 70g, ethanol 60g, finasteride 4g, micropowder silica gel 50g, chitosan 20g, Ethyl cellulose 60g, low-substituted hydroxypropyl cellulose 10g, magnesium stearate 5g, mannitol 2g. According to the method above, 1000 sustained-release tablets of this example were prepared, each containing about 4 mg of finasteride.
[0039]The tablets prepared in this embodiment are easy to demould, and 99.99% of the tablets have a uniform and smooth surface without cracking or loosening. Take six tablets and test their average disintegration speed in water is 28s.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com