Preparation method of high-purity ethyl 4-chloro-3-hydroxybutyrate
A technology of ethyl hydroxybutyrate and ethyl chloroacetoacetate, which is applied in the field of preparation of high-purity ethyl 4-chloro-3-hydroxybutyrate, can solve the problems of many impurities and low purity, so as to improve the purity and reduce the Concentration temperature, the effect of reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
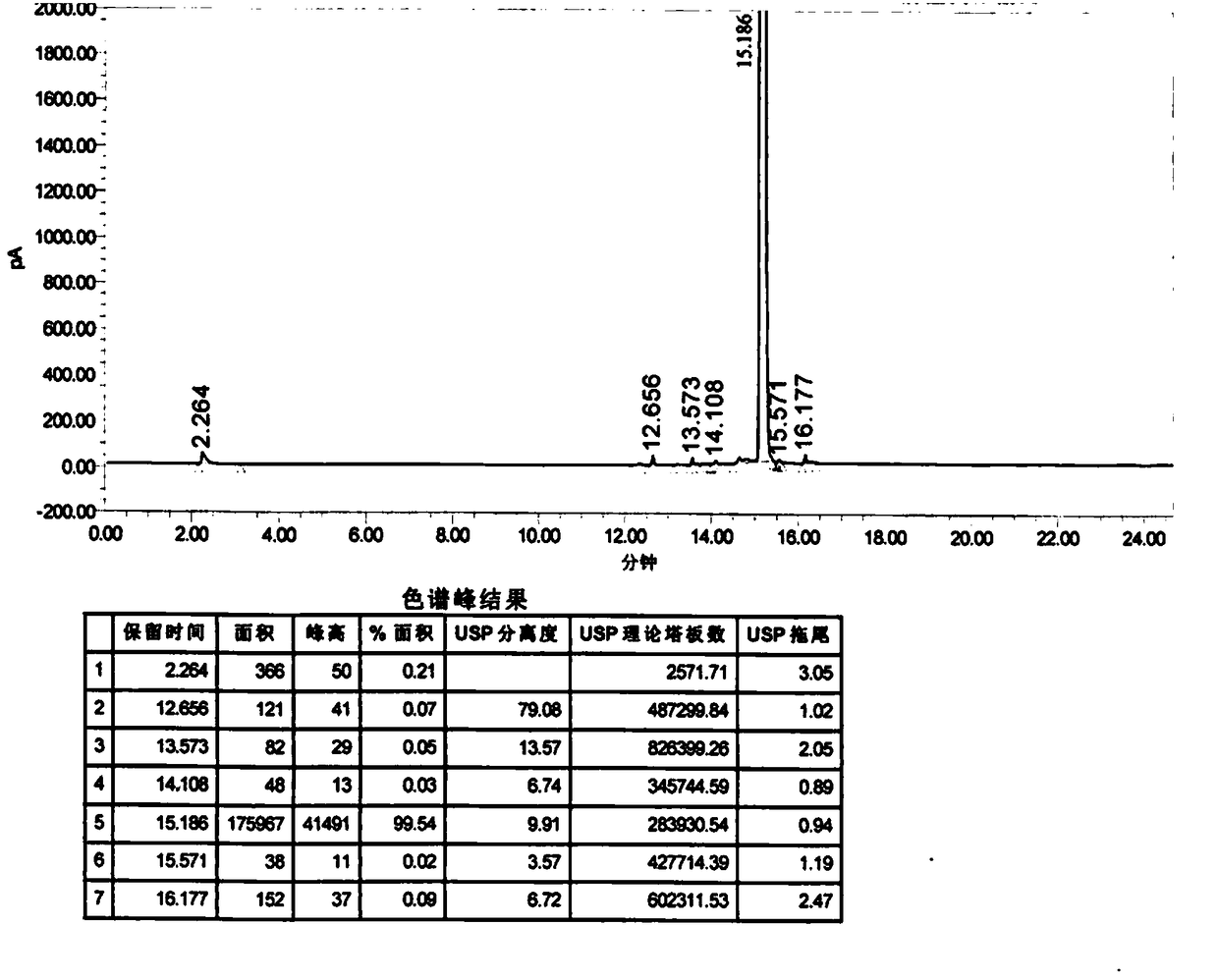
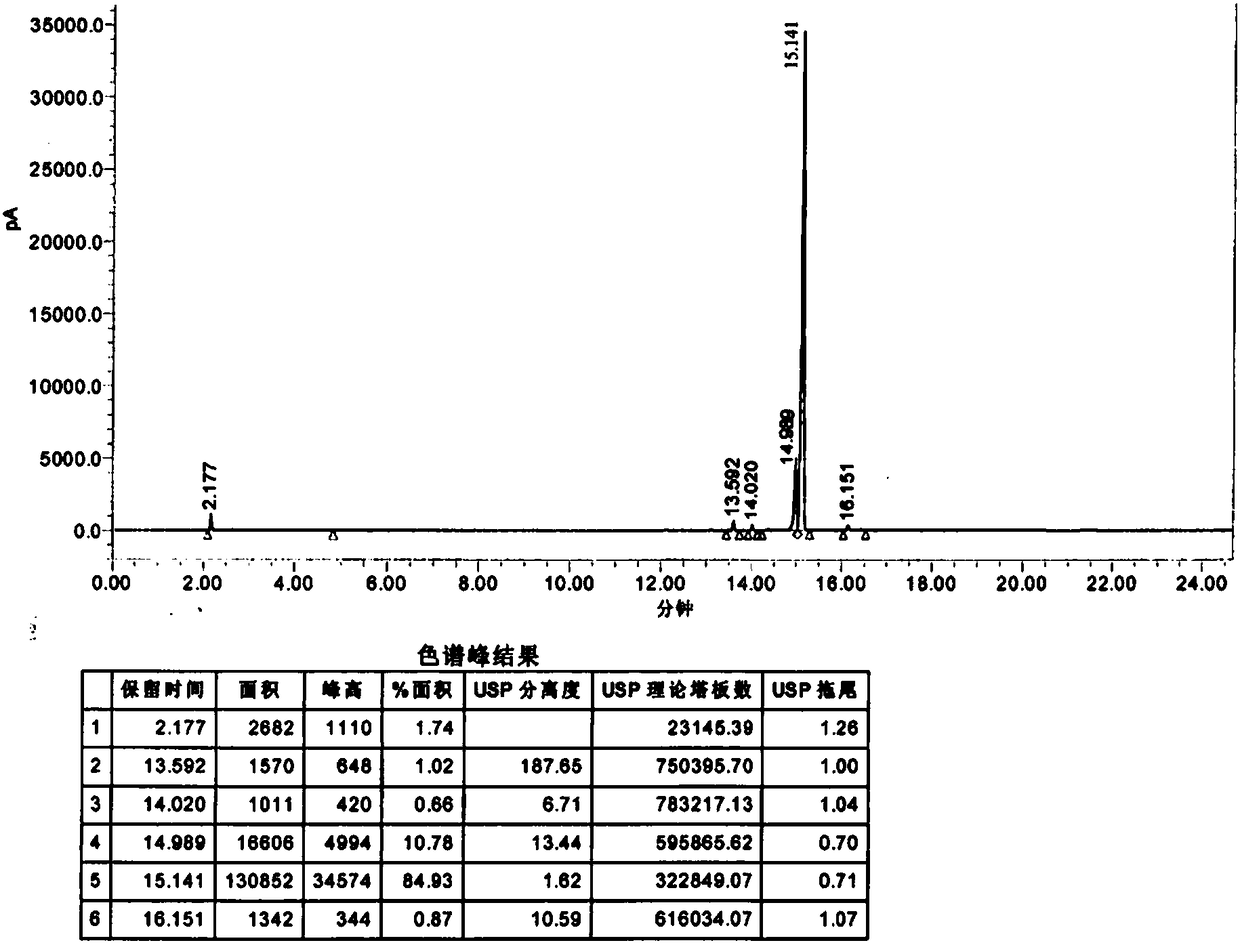
Image
Examples
Embodiment 1
[0029] Add 50g of ethyl 4-chloroacetoacetate and 300ml of absolute ethanol into a 1L reaction flask, and cool down to -10°C. Add 3.0 g of sodium borohydride several times, and control the temperature at -10 to -5°C. After the addition, react at -10 to -5°C for 1 hour. After the reaction, add 10 g of anhydrous sodium sulfate, adjust the pH to neutral with glacial acetic acid, about 4 ml, and stir for 10 minutes.
[0030] Suction filtration to remove salt, the filtrate was concentrated under reduced pressure until no distillate was evaporated, and the temperature was lowered to 25-30°C. Add 200ml of dichloromethane and stir for 10 minutes, wash with 40*2ml of purified water twice, add 20g of anhydrous sodium sulfate to the organic phase and dry for 2 hours. Dichloromethane was distilled off by concentration to obtain crude 4-chloro-3-hydroxybutyric acid ethyl ester. Continue vacuum distillation to obtain the fine product of ethyl 4-chloro-3-hydroxybutyrate with a purity of 99...
Embodiment 2
[0032] Add 50g of ethyl 4-chloroacetoacetate and 300ml of absolute ethanol into a 1L reaction flask, and cool down to -10°C. Add 4.5 g of sodium borohydride several times, and control the temperature at -10 to -5°C. After the addition, react at -10 to -5°C for 1 hour. After the reaction, add 10 g of anhydrous sodium sulfate, adjust the pH to neutral with glacial acetic acid, about 4 ml, and stir for 10 minutes.
[0033] Suction filtration to remove salt, the filtrate was concentrated under reduced pressure until no distillate was evaporated, and the temperature was lowered to 25-30°C. Add 200ml of dichloromethane and stir for 10 minutes, wash with 40*2ml of purified water twice, add 20g of anhydrous sodium sulfate to the organic phase and dry for 2 hours. Dichloromethane was distilled off by concentration to obtain crude 4-chloro-3-hydroxybutyric acid ethyl ester. Continue vacuum distillation to obtain the fine product of ethyl 4-chloro-3-hydroxybutyrate with a purity of 99...
Embodiment 3
[0035] Add 50g of ethyl 4-chloroacetoacetate and 300ml of absolute ethanol into a 1L reaction flask, and cool down to -10°C. Add 7.8 g of sodium borohydride several times, and control the temperature at -10 to -5°C. After the addition, react at -10 to -5°C for 1 hour. After the reaction, add 10 g of anhydrous sodium sulfate, adjust the pH to neutral with glacial acetic acid, about 4 ml, and stir for 10 minutes.
[0036] Suction filtration to remove salt, the filtrate was concentrated under reduced pressure until no distillate was evaporated, and the temperature was lowered to 25-30°C. Add 200ml of dichloromethane and stir for 10 minutes, wash with 40*2ml of purified water twice, add 20g of anhydrous sodium sulfate to the organic phase and dry for 2 hours. Dichloromethane was distilled off by concentration to obtain crude 4-chloro-3-hydroxybutyric acid ethyl ester. Continue vacuum distillation to obtain the fine product of ethyl 4-chloro-3-hydroxybutyrate with a purity of 99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com