Feline infectious rhinoconjunctivitis and feline panleukopenia dual vaccine and preparation method thereof
A technology for leukopenia and rhinoconjunctivitis, which is applied in the fields of microorganisms and veterinary biopharmaceuticals, can solve the hidden dangers of attenuated vaccines, stimulate cellular immunity, and fail to immunize vaccines, and achieve good immune prevention effects, good safety, and immunogenicity high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
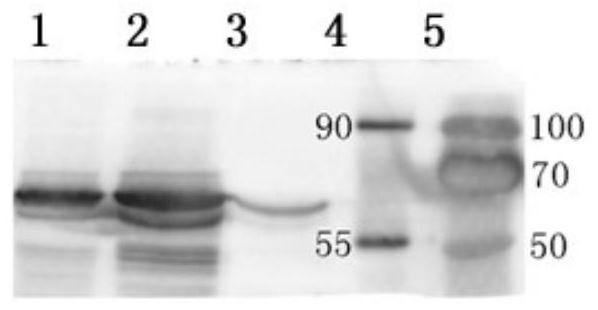
[0030] Example 1 Preparation and identification of feline calicivirus virus-like particles
[0031]After extracting RNA from the FCV-JL2 strain, reverse transcription, and PCR sequencing, the VP1 sequence was obtained and optimized according to the insect NEWII cell preference. The codon preference index was adjusted from 0.47 to 0.98, and the GC content was adjusted from 42.6% to 57.6 %, and remove unfavorable cis-acting elements, remove mRNA prone to neck-loop regions, etc. Send the optimized sequence to the company for gene synthesis. For the optimized sequence, use primers to amplify the VP1 gene, and connect it into the pFastBac Dual vector through NotI-HindIII and SmaI-XhoI, respectively, to obtain a transfer vector carrying double-copy foreign fragments. The transfer vector was transformed into DH10Bac competent for recombination, and the recombinant baculovirus genome Bacmid carrying double-copy exogenous fragments was obtained. After Bacmid was transfected into Sf9 ...
Embodiment 2
[0039] Example 2 Preparation and Identification of Feline Panleukopenia Virus Virus-Like Particles
[0040] After sequencing the extracted genome of the FPV strain, the VP2 gene sequence was obtained and optimized according to the insect NEW II cell preference. The codon preference index was adjusted from 0.37 to 0.87, and the GC content was adjusted from 35.1% to 53.0%. Send the optimized sequence to the company for gene synthesis. For the optimized sequence, use primers to amplify the VP2 gene, and connect them into the pFastBac Dual vector through NotI-HindIII and XhoI-NheI, respectively, to obtain transfer vectors carrying double-copy foreign fragments. The transfer vector was transformed into DH10Bac competent for recombination, and the recombinant baculovirus genome Bacmid carrying double-copy exogenous fragments was obtained. After Bacmid was transfected into Sf9 cells, the recombinant baculovirus was rescued. Infecting Sf9 cells with recombinant baculovirus can produ...
Embodiment 3
[0042] Embodiment 3 cat immunity test
[0043] The above-mentioned recombinant baculoviruses were respectively inoculated into suspended Sf9 cells at MOI=0.1, cultured for 4 days, and then harvested. After freeze-thawing the Sf9 cells inoculated with FCV antigen once, centrifuge at 3000rpm for 30min to obtain the supernatant, which is the harvest solution containing FCV virus-like particles. The protein concentration was determined by BCA method and was 4.9 mg / ml. Centrifuge the Sf9 cells inoculated with FPV antigen directly at 3000rpm for 30min, discard the supernatant, and wash the cells with an equal volume of 25mM NaHCO 3 Suspend, act on ice for 30 minutes, and centrifuge at 3000 rpm for 30 minutes to obtain the supernatant, which is the harvest solution containing FPV virus-like particles. Use pig blood for the determination of hemagglutination titer, HA is 1:2 16 .
[0044] Mix the FCV virus-like particles and FPV virus-like particles prepared above at a volume of 3:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com