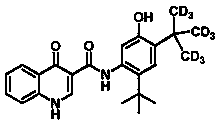
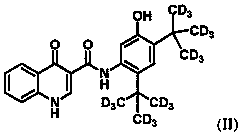
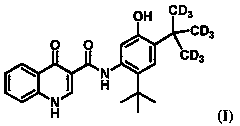
Administration of deuterated cftr potentiators
A technology for preparation and tablet, applied in 0010] The present invention relates to the new field of ivacaftor derivatives, which can solve problems such as unpredictable effects and inconsiderations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] Such methods of preparation include the step of bringing into association the molecule to be administered with ingredients such as the carrier which constitute one or more accessory ingredients. In general, the compositions are prepared by uniformly and intimately bringing into association the active ingredient with liquid carriers, liposomes or finely divided solid carriers or both, and then, if necessary, shaping the product.
[0065] In certain embodiments, the compounds are administered orally. Compositions of the present invention suitable for oral administration may be presented as discrete units such as capsules, sachets or tablets each containing a predetermined amount of the active ingredient; powder or granules; in an aqueous or non-aqueous liquid as a solution or suspension; an oil-in-water liquid emulsion; a water-in-oil liquid emulsion; packaged in liposomes; or as a bolus, etc. Soft gelatin capsules may be used to contain this suspension, which may be adv...
Embodiment 1
[0094] Example 1. Phase 1 single ascending dose (SAD) clinical trial
[0095] Ten healthy male and female volunteers participated in a single ascending dose study of 3 doses of CTP-656 (75, 150 and 300 mg), cross-comparing 150 mg of CTP-656 and 150 mg of Kalydeco® (trade name Ivaca support) ( Figure 5 ). Each dose of CTP-656 was given as an aqueous suspension, and Kalydeco as a tablet. All doses of CTP-656 and Kalydeco were administered within 30 minutes of the start of a high-fat breakfast. There was a 7 day washout between doses. The objectives of the study were to compare the pharmacokinetics of single ascending doses (75, 150, and 300 mg) of CTP-656, to compare the pharmacokinetics of single doses of 150 mg CTP-656 and 150 mg Kalydeco, and to evaluate the pharmacokinetics of CTP-656. Safety and Tolerability.
[0096] Overall, CTP-656 administered as a single dose at doses of 75 mg, 150 mg, and 300 mg (following a high-fat meal) was generally well tolerated in healthy...
Embodiment 2
[0107] Example 2. Relationship of Parent and Metabolite Pharmacokinetic Profiles
[0108] Such as image 3 As shown in , after a single dose, deuteration significantly affects the metabolism of the deuterated ivacaftor analog CTP-656 compared with ivacaftor. Ivacaftor, CTP-656 and their metabolites are shown in Image 6 middle. The production of metabolites D8-M1 and D6-M6 from CTP-656 was significantly reduced relative to the production of metabolites M1 and M6 from ivacaftor. Therefore, compared to the ivacaftor to M1 ratio of 0.58, the AUC of CTP-656 / D8-M1 0-24hr The parent to M1 ratio of 2.0. CTP-656 / D8-M1 C max and C 24hr The maternal to M1 ratios were 2.1 and 2.2, respectively. Further, compared with the ratio of ivacaftor to M6 of 1.5, the AUC of CTP-656 / D6-M6 0-24hr The parent to M6 ratio was 4.0. CTP-656 / D6-M6 compared with 1.4 and 0.97 for ivacaftor / M6 max and C 24hr The parent to M6 ratios were 4.3 and 2.5, respectively. Such as image 3 As seen in (a),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com