A kind of isomer detection method of camphorsulfonic acid or its salt
A technology of camphorsulfonic acid and detection method, which is applied in the field of detection, can solve problems such as the detection and analysis of camphorsulfonic acid or its salt, and achieve the effects of smooth baseline, good separation and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
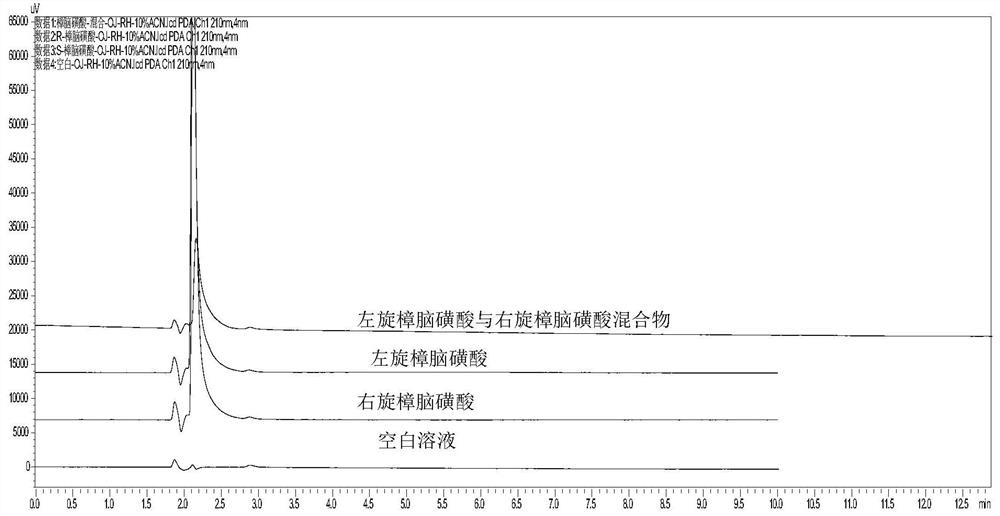
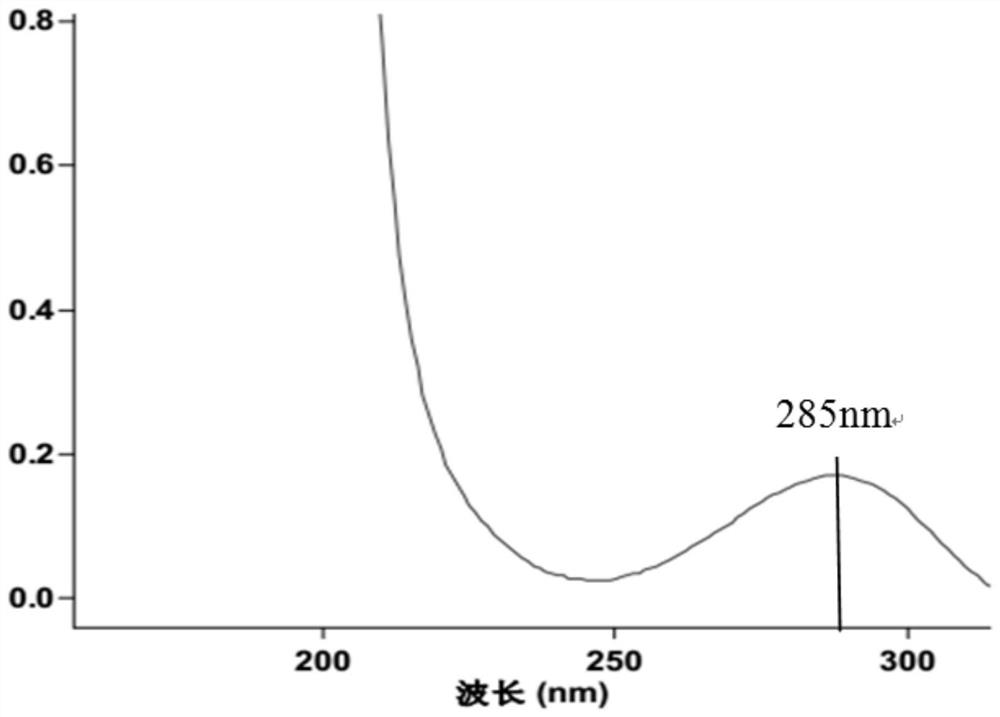
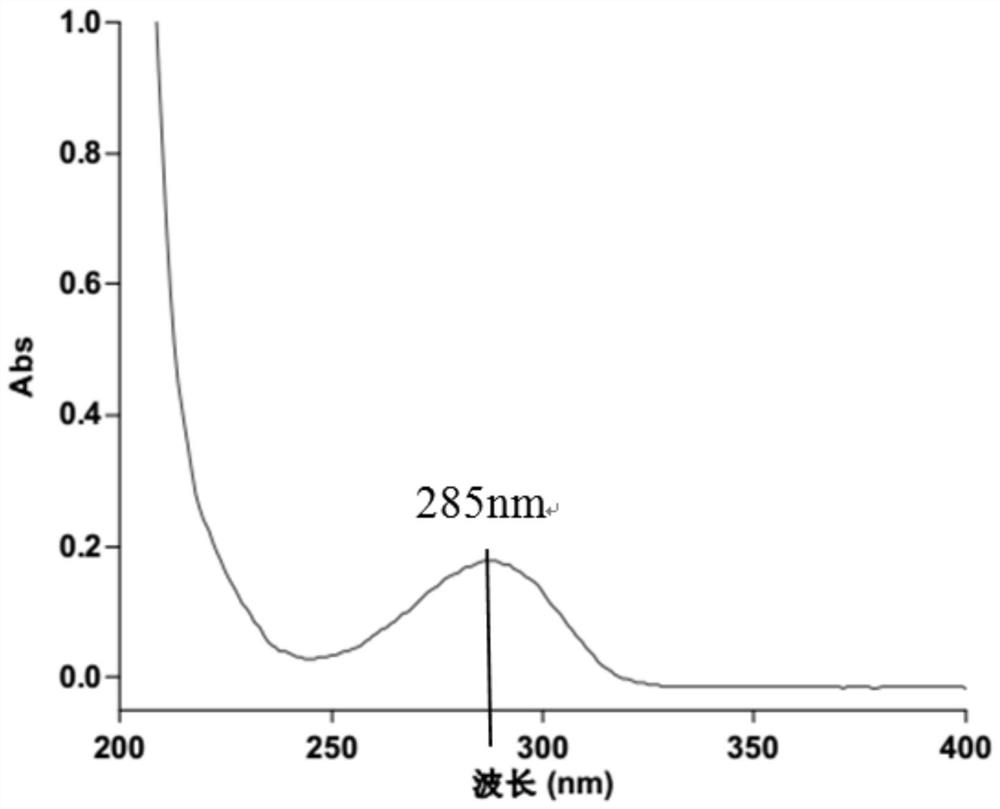
[0037] Take an appropriate amount of L-camphorsulfonic acid test product, accurately weighed, dissolve and dilute with methanol to a solution containing about 5 mg of L-camphorsulfonic acid per 1 ml, as the test solution; accurately pipette an appropriate amount of the test solution, and dilute with methanol A solution containing about 50 μg of levocamphorsulfonic acid per 1 ml was used as a control solution. In addition, accurately weigh an appropriate amount of the reference substance of camphorsulfonic acid racemate, dissolve it with methanol and dilute it into a solution containing about 2.5 mg each of L-camphorsulfonic acid and D-camphorsulfonic acid per 1 ml, as a system suitability solution. According to the test of high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512), the silica gel with quinidine derivatives bonded on the surface is used as filler (Daicel Chiralpak ZWIX (-) 250mm×4mm, 3μm or equivalent chromatographic colu...
Embodiment 2
[0039] Methodological Study on Isomers of Levocamphorsulfonic Acid
[0040] 1. Analytical method establishment
[0041] 1.1 Overview
[0042] L-camphorsulfonic acid is a chiral resolving agent used in the synthesis of voriconazole, which plays a key role in the formation of the chiral center of voriconazole. From the synthesis route of L-camphorsulfonic acid, it can be seen that the enantiomers of L-camphorsulfonic acid are more likely to remain than D-camphorsulfonic acid. In combination with the opinions issued by the Food and Drug Administration on voriconazole, the optical purity of L-camphorsulfonic acid is controlled by a specific method requirements. An HPLC method was established to control the content of enantiomers in L-camphorsulfonic acid.
[0043] 1.2 Screening and optimization
[0044] 1.2.1 Method preliminary screening
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com