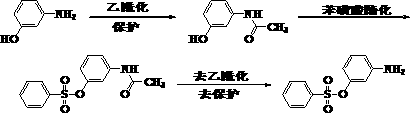
Continuous synthesis method of m-acetamido phenol
A technology of m-acetamide and phenol, which is applied in the field of continuous synthesis of m-acetamidophenol, can solve the problems of poor safety, three wastes, and high technical cost, and achieve improved safety, less by-products, and high safety of the synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1 m-acetamidophenol
[0028] 91.5 g (0.61 mol) m-acetamidoaniline, 270 g (2.70 mol) 98% sulfuric acid and 1200 mL water, stirred evenly to make solution A, 42.8 g (0.62 mol) sodium nitrite and 180 mL water, stirred evenly to make solution A Solution B. Solution A and solution B were fed by metering pump A and metering pump B respectively, the flow rate ratio of metering pump A and metering pump B was controlled to be 1:7.1, and the residence time of the reaction liquid in the tubular reactor was controlled to be 25 s. The whole tubular reactor is a copper tube with an inner diameter of 16 mm and a length of 2 m, and the tube is immersed in an oil bath with a constant temperature of 120 °C. The reaction solution flowed out into a four-necked flask with stirring and cooling, extracted with 3x200 mL of methyl isobutyl ketone at an extraction temperature of 20-35°C, washed with water, and dried over anhydrous sodium sulfate. Filtration and prec...
Embodiment 2
[0029] The synthesis of embodiment 2 m-acetamidophenol
[0030] 91.5 g (0.61 mol) m-acetamidoaniline, 180 g (1.80 mol) 98% sulfuric acid and 1000 mL water, stirred evenly to make solution A, 44.2 g (0.64 mol) sodium nitrite and 160 mL water, stirred evenly to make solution A Solution B. Solution A and solution B were fed by metering pump A and metering pump B respectively, the flow rate ratio of metering pump A and metering pump B was controlled to be 1:6.3, and the residence time of the reaction liquid in the tubular reactor was controlled to be 80 s. The tubular reactor consists of two parts. The first part of the reaction section is a copper tube with an inner diameter of 4 mm and a length of 10 m. m, the pipe was submerged in an ice-water bath. The reaction solution was collected, extracted with 4x150 mL of toluene at an extraction temperature of 50-60°C, washed with water, and dried over anhydrous sodium sulfate. Filtration and precipitation yielded 77.7 g of crude m-a...
Embodiment 3
[0031] The synthesis of embodiment 3 m-acetamidophenol
[0032] 91.5 g (0.61 mol) m-acetamidoaniline, 360 g (3.60 mol) 98% sulfuric acid and 800 mL water, stirred evenly to make solution A, 45.0 g (0.65 mol) sodium nitrite and 110 mL water, stirred evenly to make solution A Solution B. Solution A and solution B were fed by metering pump A and metering pump B respectively, the flow rate ratio of metering pump A and metering pump B was controlled to be 1:8.1, and the residence time of the reaction liquid in the tubular reactor was controlled to be 220 s. The whole tubular reactor is a copper tube with an inner diameter of 6 mm and a length of 40 m, and the tube is immersed in an oil bath at a constant temperature of 70 °C. The reaction solution flowed into a four-neck flask with stirring and cooling, extracted with 4x180 mL of cyclohexane at an extraction temperature of 45-55°C, washed with water, and dried over anhydrous magnesium sulfate. Filtration and precipitation yielded...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com