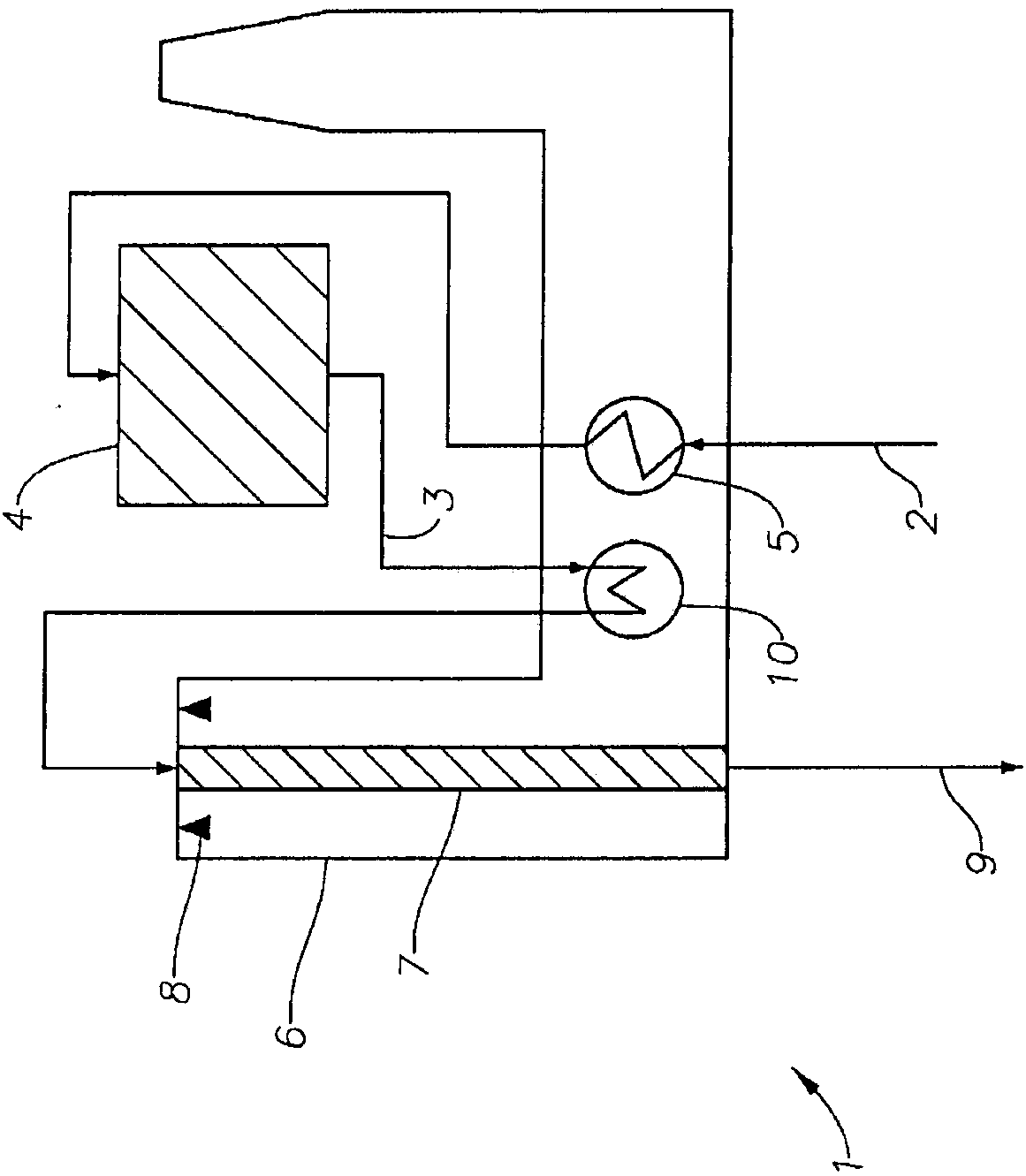
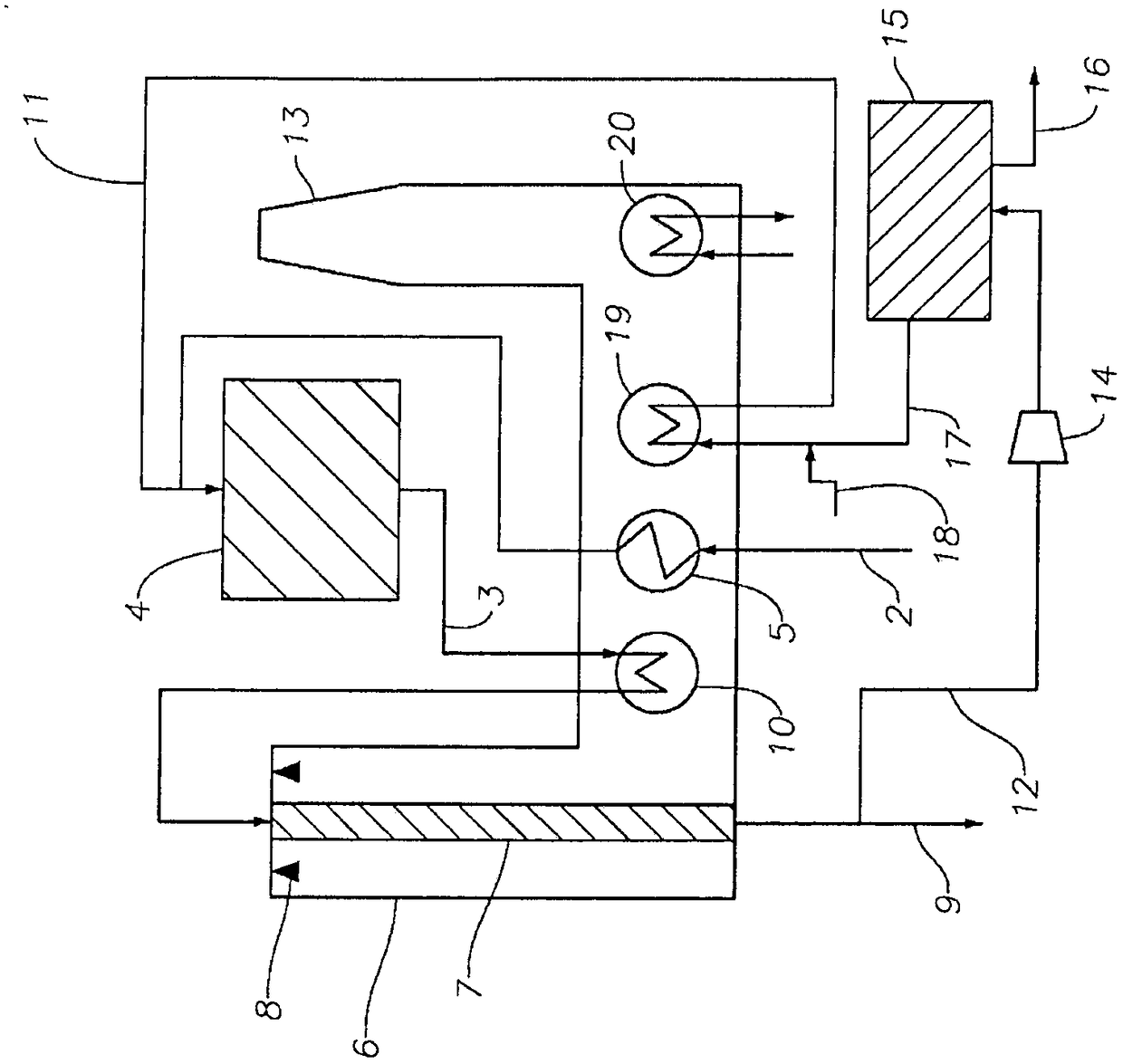
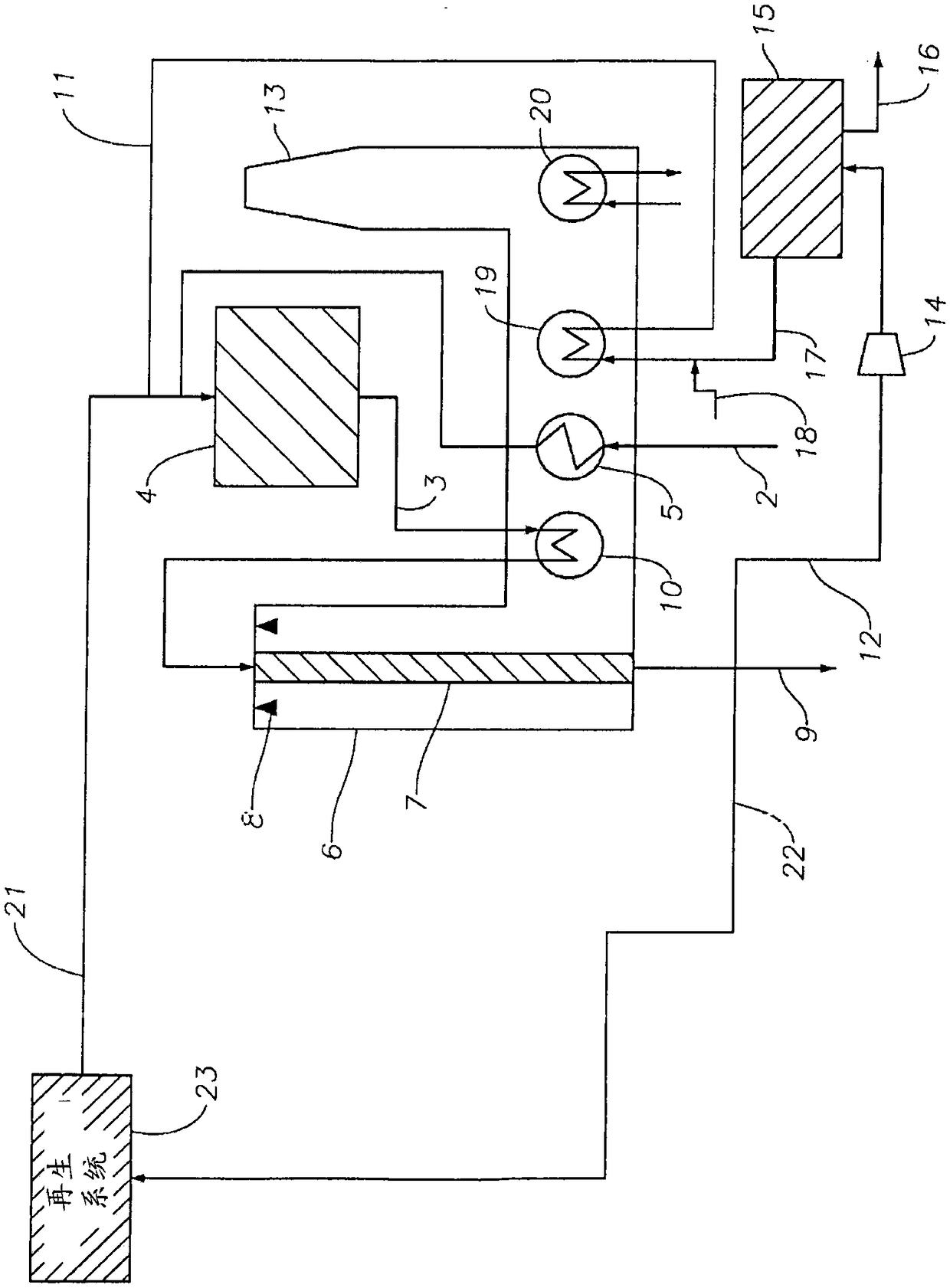
Processes and systems for converting hydrocarbons to cyclopentadiene
A technology for the conversion of cyclopentadiene and hydrocarbons, which is applied in the field of reactors and can solve problems such as coking and loss of catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-Z
[0321] Synthesis of embodiment 1-ZSM-5 catalyst composition
[0322] Consists of 10,000g deionized (DI) water, 600g 50% NaOH solution, 25g 45% sodium aluminate solution, 730g n-propylamine 100% solution, 80g ZSM-5 seed crystal and 3,190g Ultrasil PM TM The synthetic mixture prepared with modified silica at approximately 20.3% solids was mixed in a 5 gallon vessel and then added to a 5 gallon autoclave after mixing. The synthesis mixture has the following molar composition:
[0323]
[0324] The synthesis mixture was mixed and reacted at 230°F (110°C) for 72 hours at 250 rpm. The resulting product was filtered and washed with deionized water, then dried in an oven at about 250°F (121°C) overnight. The XRD pattern (not shown) of the as-synthesized material showed pure phases typical of the ZSM-5 topology and that the material consisted of a mixture of large crystals of about 2 microns in size. A portion of the as-synthesized crystals were converted (for characterization)...
Embodiment 2
[0326] Embodiment 2-catalyst composition performance evaluation
[0327] The catalyst composition of Example 1 (0.5 g) was physically mixed with quartz (1.5 g, 60-80 mesh) and charged into the reactor. The catalyst composition was dried under He (100 mL / min, 30 psig (207 kPa), 250 °C) for 1 h, then 2 (200 mL / min, 30 psig (207 kPa), 500° C.) for 1 hour. Then use n-pentane, H 2 And the feed of the balance He, typically at 550-600 ° C, 5.0 psia (35 kPa-a) C 5 h 12 , 1.0 mol H 2 :C 5 h 12 , 14.7h -1 WHSV and total 30 psig (207 kPa) test catalyst composition. At 550-600°C by using H 2 After an initial test of 5 hours of treatment (200 mL / min, 30 psig (207 kPa), 650°C), the catalyst composition was tested for stability and regeneration performance, and then retested for performance at 600°C.
[0328] Cyclopentadiene and three equivalents of hydrogen are produced by dehydrogenation and cyclization of n-pentane (Equation 1). This is accomplished by passing n-pentane throu...
Embodiment 3
[0336] Example 3 - Reactor Performance Modeling
[0337] The above data set and similar experimental data were used to guide in Invensys Systems Inc. PRO / II 9.1.4 (for Examples 3A-3H) and Invensys Systems Inc. PRO / II 9.3.4 (for Examples 3I-3N ) to build models to estimate performance under various commercially relevant operating conditions and different reactor configurations. Depending on the specifics of the modeling, variations in the results can occur, but the models still show the relative benefits of the invention. Many modifications and changes are possible, and it is understood that within the scope of the appended claims, the invention may be practiced otherwise than as specifically described herein. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com