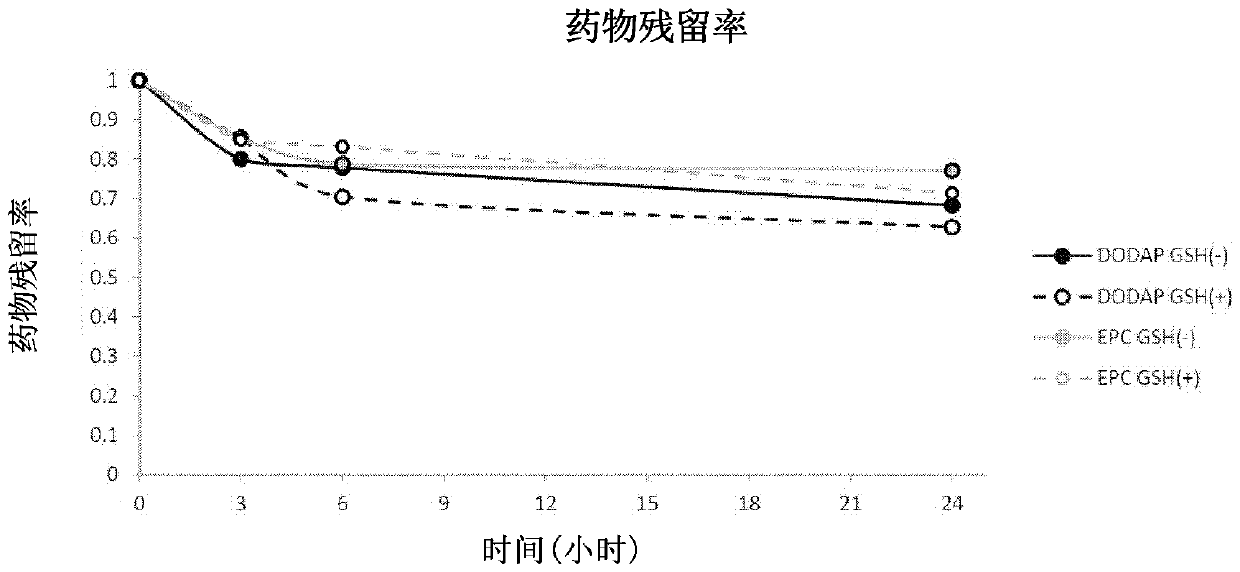
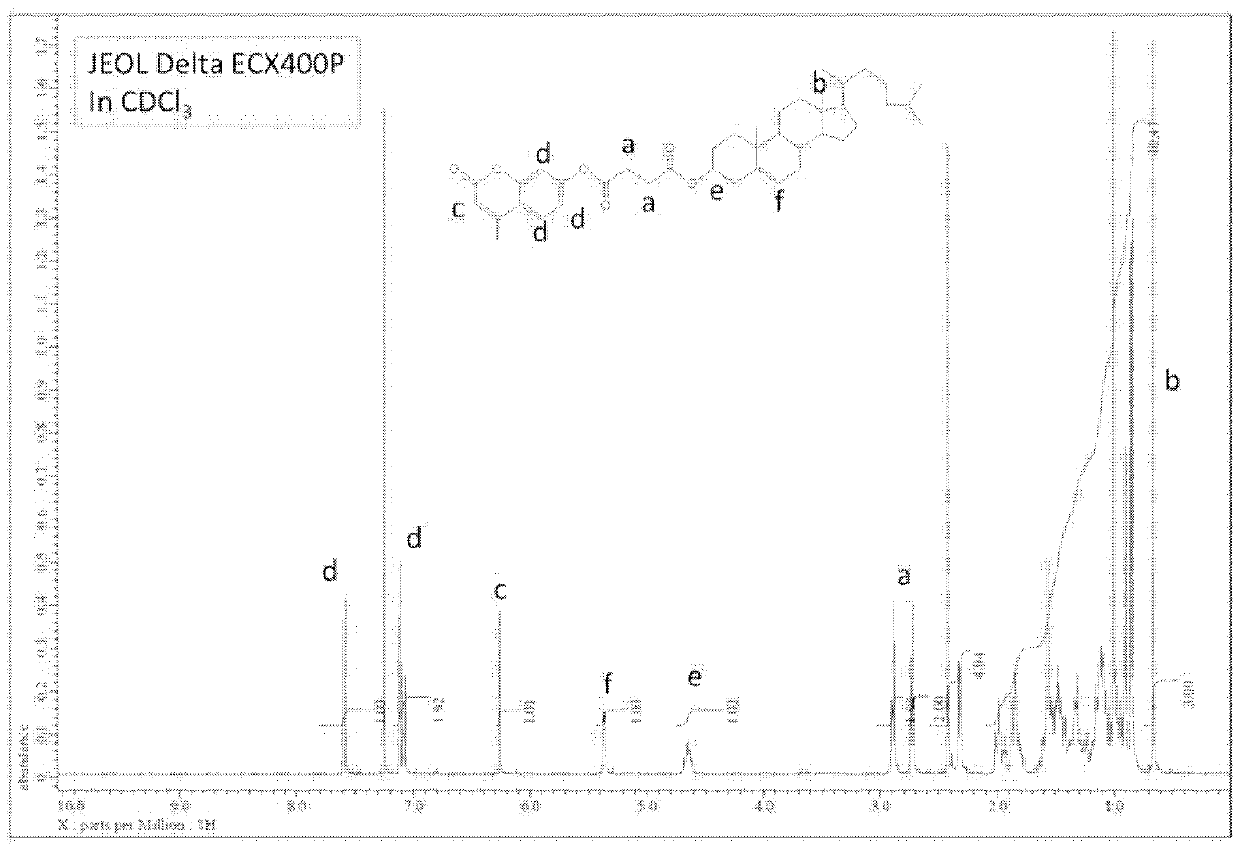
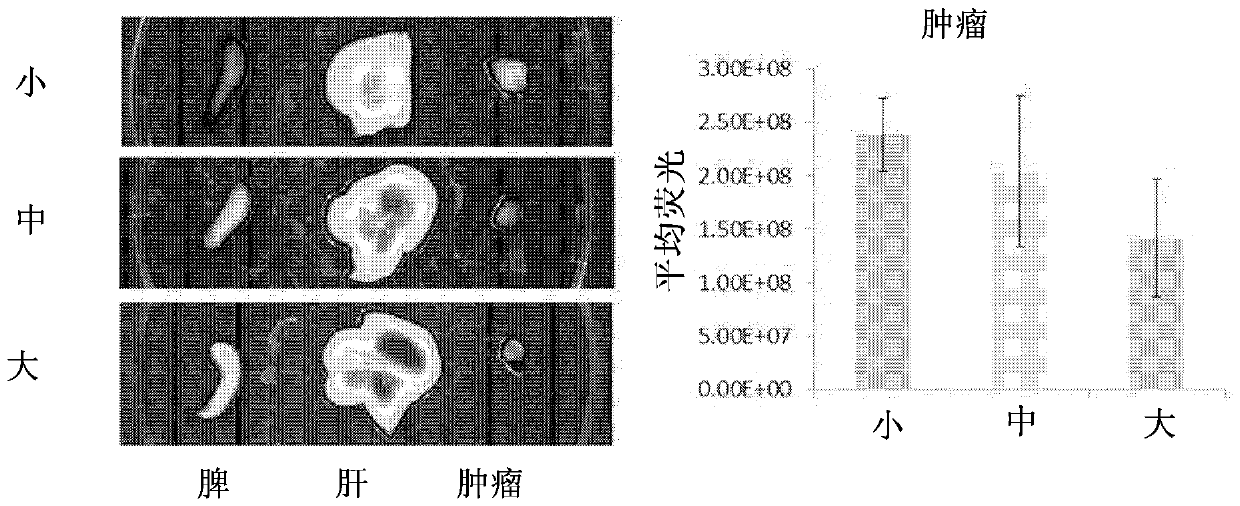
O/w type emulsion
An emulsion, independent technology, applied in the direction of emulsion delivery, allergic diseases, metabolic diseases, etc., to achieve the effect of high accumulation and high blood retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0272] The poorly water-soluble drug can be internally encapsulated in the O / W type emulsion of the present invention by performing emulsification using a lipid solution containing the poorly water-soluble drug dissolved therein in the preparation of the above O / W type emulsion. The present invention also provides the aforementioned O / W emulsion of the present invention in which a poorly water-soluble drug is encapsulated.
[0273] In the present invention, a poorly water-soluble drug refers to a drug having a water / octanol partition coefficient LogPow of not less than 4 for evaluating the hydrophobicity of a compound. Examples of such poorly water-soluble drugs include tacrolimus (LogPow: 4.79), ursodeoxycholic acid (LogPow: 4.76), oxicaine (LogPow: 4.38), simvastatin (LogPow: 4.72), ethinylestradiol Alcohol (LogPow: 4.11), Clotrimazole (LogPow: 4.93), Zaltoprofen (LogPow: 4.25), Betamethasone Valerate (LogPow: 4.14), Pentazocin (LogPow: 4.15), Iohexacic Acid (LogPow: 4.32),...
Embodiment 1
[0306] [Example 1] Preparation of fine O / W type emulsion
[0307] As cationic lipid, use B-2, B-2-5, O-C3M, B-2-3, TS-P4C2 or L-PZ4C2, prepare cationic lipid:DOPE:cholesterol=3:4: O / W type emulsion with a composition of 3 (molar ratio). In this preparation, as a model drug, 4-methylumbelliferone (10 mol%) was used, and as PEG lipids, DMG-PEG2000 (10 mol%) and DSG-PEG2000 (2.5 mol%) were used, and prepared 1.0 mM (based on lipid concentration) emulsion.
[0308] An ethanol solution containing 1000 nmol of the total amount of cationic lipid, DOPE, and cholesterol was prepared, and a model drug and PEG lipid were added in the above amounts. Ethanol was added so that the total amount was 400 μL, and the mixture was left standing at ice temperature for 15 minutes. Similarly ice-cold malic acid buffer (20 mM, pH 3.0, 30 mM NaCl) (400 μL) was mixed within 3 seconds under vortexing. PBS (NISSUI) (3200 μL) was immediately added thereto. The mixture was subjected to ultrafiltration...
Embodiment 2
[0319] [Example 2] Effect of pH on O / W Type Emulsion
[0320] As cationic lipid, use B-2 to prepare cationic lipid:DOPE:cholesterol=3:4:3 (molar ratio) or cationic lipid:DOPC:cholesterol=3:4:3 (molar ratio) The composition of the O / W type emulsion. In this preparation, as PEG lipid, DMG-PEG2000 (15 mol%) was used, and an emulsion of 0.5 mM (based on lipid concentration) was prepared.
[0321] An ethanol solution containing 500 nmol of the total amount of B-2, DOPE, and cholesterol was prepared, and PEG lipid was added in the above amount. Ethanol was added so that the total amount was 200 μL, and the mixture was left standing at ice temperature for 15 minutes. Similarly ice-cold malic acid buffer (20 mM, pH 3.0-pH 5.0) (200 μL) was mixed within 3 seconds under vortexing. PBS (NISSUI) (3600 μL) was immediately added thereto. The mixture was subjected to ultrafiltration using an Amicon Ultra (Millipore). Centrifugation conditions were 1000G, 25°C. The operation including a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com