Amphiphilic hyperbranched polymer and its preparation method and application
A technology of amphiphilic hyperbranched and hyperbranched polymers, applied in the field of biochemistry, can solve the problems of ineffective stimulation of antigen presentation, anaphylactic shock, and short half-life of soluble peptides, so as to improve the severity of the disease and enhance the immunogen Sexuality and recovery of walking ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic method of embodiment 1, hyperbranched polymer
[0030] The synthetic method of hyperbranched polymer comprises the steps:
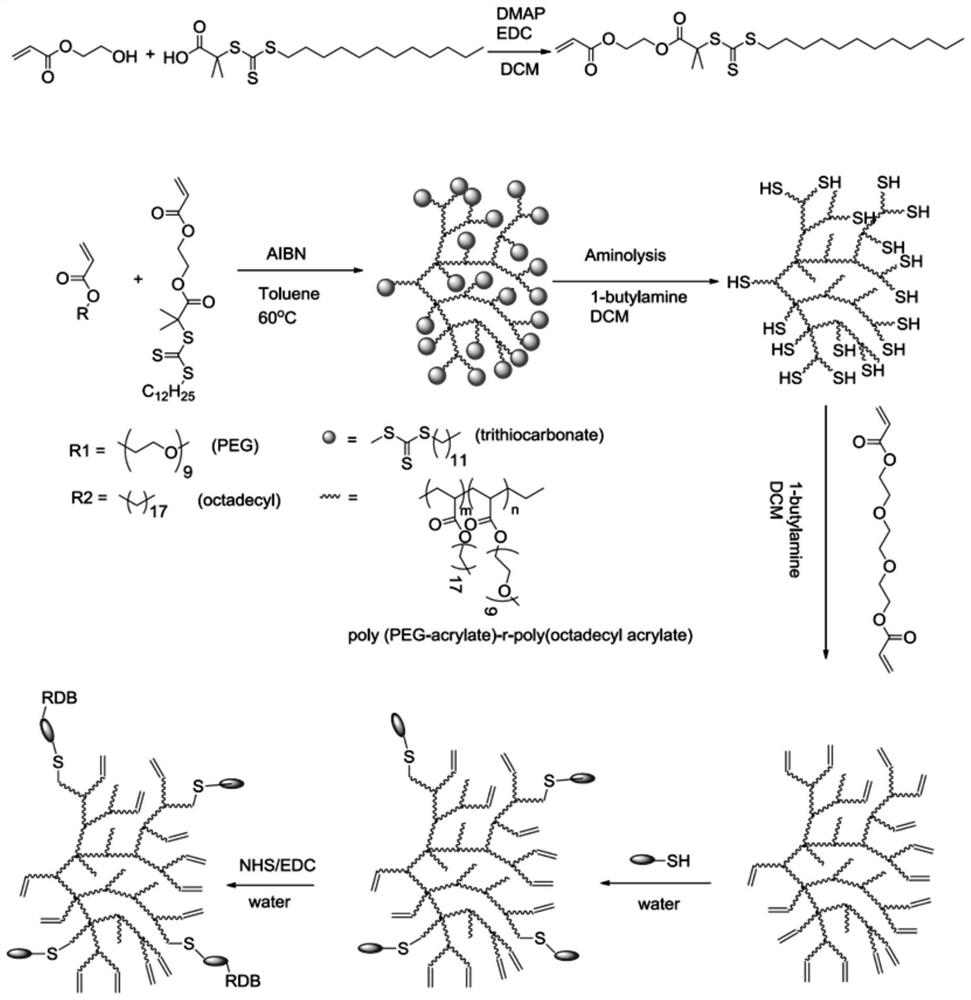
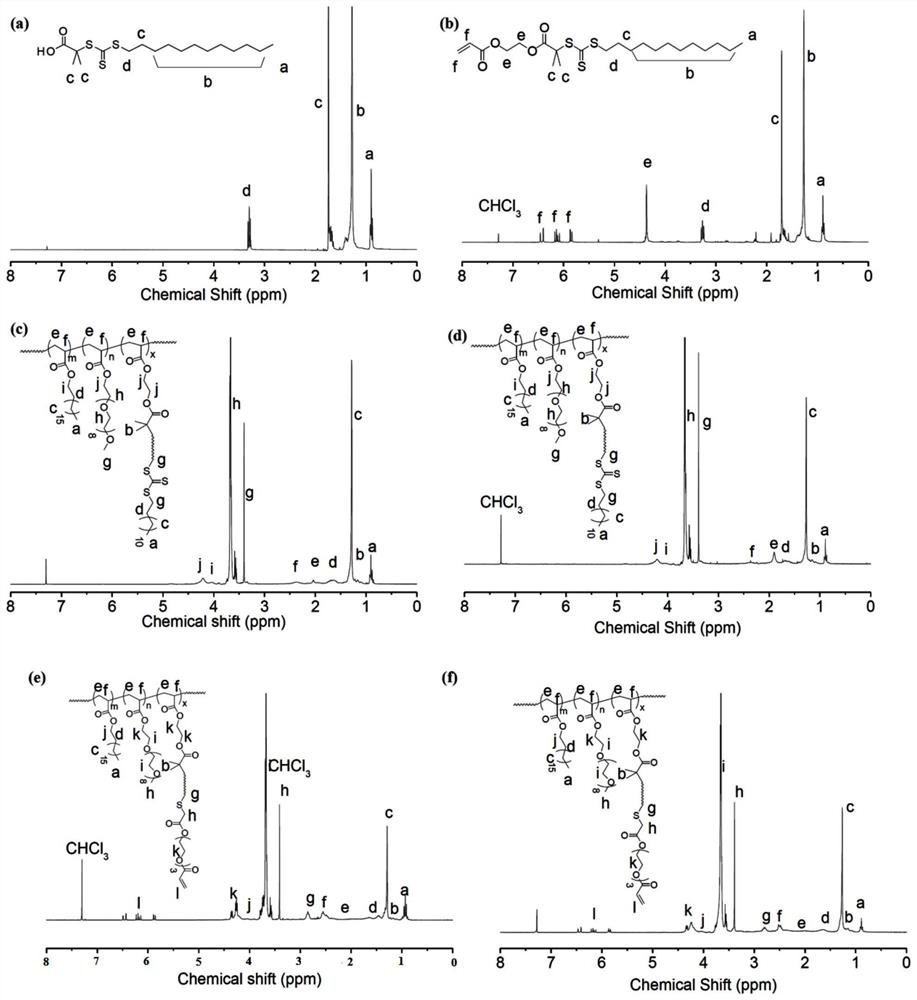
[0031] (1) Synthesis of S-Dodecyl-S'-(α,α'-dimethyl-α”-acetic acid)trithiocarbonate (DMATC): For its synthesis method, please refer to the use of new carbonate carboxy-terminated as three efficient RAFT agent synthesis functions Sexual polymers (Macromolecules, 2002, 35(18), pp 6754-6756).
[0032] (2) Synthesis of DMATC-acrylate: DMATC chain transfer agent and acrylate cross-linked by the esterification of DMATC catalyzed by DMAP / EDC and hydroxyethyl acrylate, the specific method is as follows: in a 25ml round bottom flask, add 1.46g DMATC ( dodecyl trithiocarbonate), 1.15g EDC hydrochloride (C 8 h 17 N 3 HCl; 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride), 0.073g DMAP (4-dimethylaminopyridine), pre-dried in vacuum in the flask at room temperature (18-25°C) Overnight; then add 1.4g of hydroxyethyl acrylate, the flas...
Embodiment 2
[0037] Embodiment 2, hyperbranched polytrithiocarbonate modification
[0038] The obtained hyperbranched polytrithiocarbonate is further decomposed into polythiol by butylamine ammonolysis reaction under the protection of nitrogen. In order to prevent hyperbranched polythiols from cross-linking through thiol oxidation, thiols were converted into thioethers by gradually adding thiolene under nitrogen protection. The specific method was as follows: 0.6 g of hyperbranched polytrithiocarbonate was dissolved in 1.5 ml DCM (dichloromethane), then add 0.5ml butylamine (~20 times), and protect with nitrogen. After 30 min, the yellow solution faded to colorless or pale yellow, then nitrogen was bubbled into the solution to remove most of the DCM and butylamine. Nitrogen degassed hexane (-10 mL) was transferred to the solution to precipitate the product. After 15 minutes, a colorless or pale yellow product was adhering to the bottom of the bottle, and most of the solvent was removed t...
Embodiment 3
[0039] Embodiment 3, preparation hyperbranched polythioether acrylate cross-linked polypeptide
[0040] Both MOG and OVA polypeptide N-terminals have cysteine, 80mg hyperbranched polymer, 2mg peptide (OVA), 0.2mg butylamine mixed with 5ml water and stirred overnight, then put the solution into a dialysis bag (molecular weight cut-off: 3500Da) Dialyzed in deionized water for 2 days, and finally, the product was freeze-dried and purified to obtain hyperbranched polymeric polypeptide 3 (Hyper-branch polymer conjugated MOG-1, HB-OVA-1) and hyperbranched polymeric polypeptide 4 (Hyper-branch polymer conjugated MOG-1) -2, HB-OVA-2).
[0041] According to the same method, the difference is that the MOG polypeptide was used to obtain hyperbranched polymeric polypeptide 1 (Hyper-branchpolymer conjugated MOG-1, HB-MOG-1) and hyperbranched polymeric polypeptide 2 (Hyper-branchpolymer conjugated MOG-2, HB-MOG-1) respectively. MOG-2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com