Method for synthesizing butyl hydroxy anisd
A technology for butyl hydroxyanisole and a synthetic method, which is applied in chemical instruments and methods, preparation of ether, preparation of ether by ester reaction, etc., can solve problems such as high production cost, high activity, and difficult separation, and achieve less reaction by-products, The effect of stable catalytic activity and simple recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
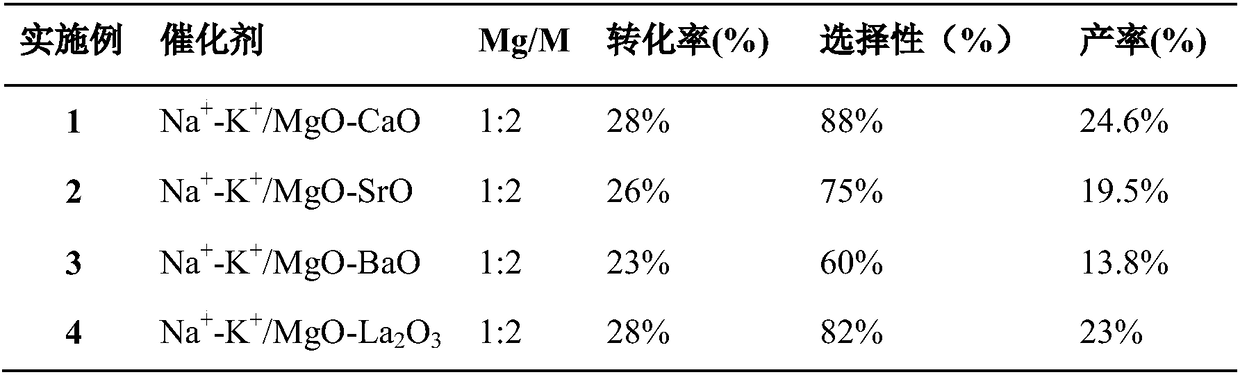
[0023] The preparation of bimetallic basic catalyst: configure the magnesium sulfate solution of 10% mass concentration, then add the nitrate solution of the second basic metal M (M=Ca, Ba, Sr or La) of 5% mass concentration, make element Mg The molar ratio to M is 1:2, and the selection of the second basic metal M is shown in Table 1. After stirring evenly, slowly add 10 times the volume of the precipitant potassium carbonate solution with a mass concentration of 2%, and keep stirring. After the precipitation is complete, filter, wash the precipitate repeatedly with double distilled water, dry at 120° C., and then calcinate at 750° C. in a muffle furnace for 15 hours. The MgO-MO obtained after calcination was pulverized and soaked overnight in 1% K and Na nitrate solution, so that the alkali metal components of K and Na in the catalyst accounted for the total content of the double alkali metal oxide MgO-MO 10mol%, the molar ratio of K and Na is 1:1. Stir and evaporate to pas...
Embodiment 5-8
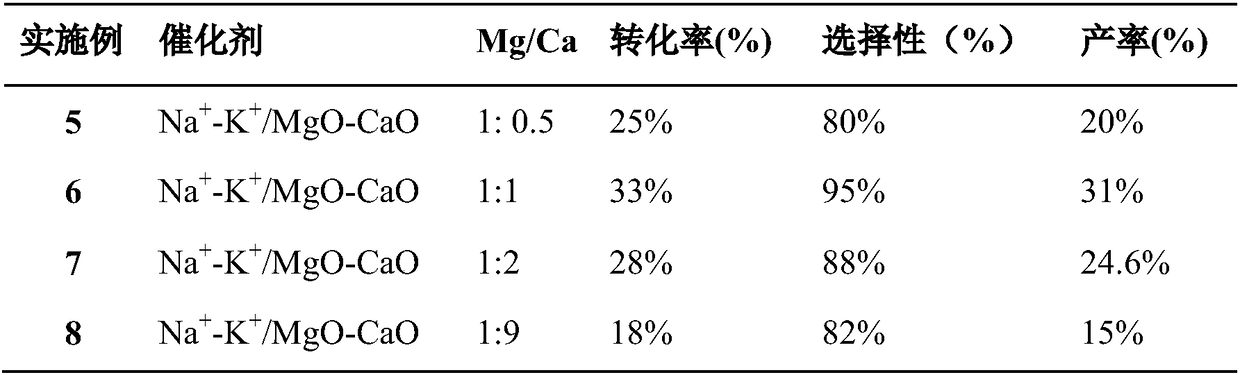
[0028] Preparation of bimetallic basic catalyst: configure magnesium sulfate solution with 10% mass concentration, and then add calcium nitrate solution with 5% mass concentration, so that the molar ratio of elements Mg and Ca is within the range of 1:0.5-9, Mg / Ca The ratio is as shown in Table 2, and slowly adding 10 times the mass concentration of the volume after stirring is the precipitating agent potassium carbonate solution of 2%, and constantly stirring. After the precipitation is complete, filter, wash the precipitate repeatedly with double distilled water, dry at 120° C., and then calcinate at 750° C. in a muffle furnace for 15 hours. The MgO-MO obtained after calcination was pulverized and soaked overnight in 1% K and Na nitrate solution, so that the alkali metal components of K and Na in the catalyst accounted for the total content of the double alkali metal oxide MgO-MO 10mol%, the molar ratio of K and Na is 1:1. Stir and evaporate to paste at 100°C, dry, extrude,...
Embodiment 9-12
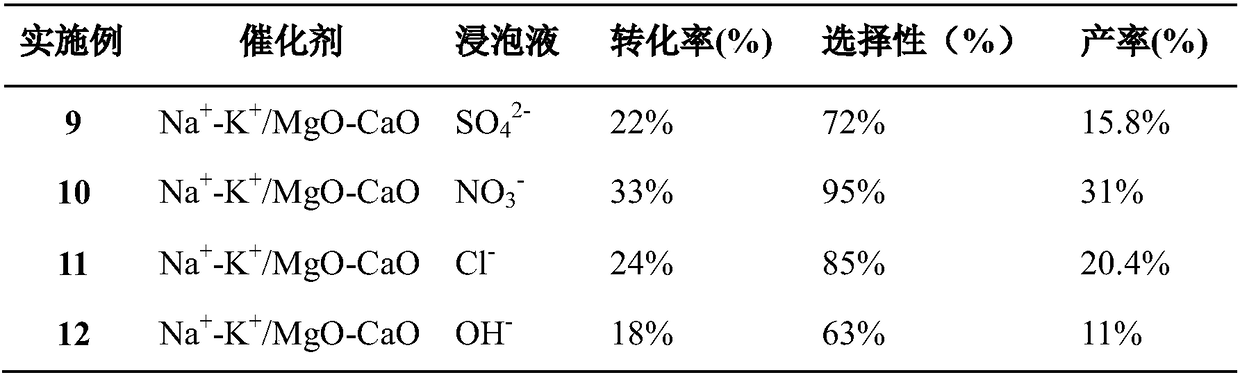
[0033] Preparation of bimetallic basic catalyst: configure 10% mass concentration of magnesium sulfate solution, then add 5% mass concentration of calcium nitrate solution, so that the molar ratio of elements Mg and Ca is 1:1, stir well and slowly add 10 times the volume The mass concentration is 2% precipitant potassium carbonate solution, and keep stirring. After the precipitation is complete, filter, wash the precipitate repeatedly with double distilled water, dry at 120° C., and then calcinate at 750° C. in a muffle furnace for 15 hours. The MgO-MO obtained after calcination was pulverized, and soaked in 1% K and Na sulfate, nitrate, chloride, hydroxide solution (as shown in Table 3) overnight, so that K and Na in the catalyst The alkali metal component accounts for 10 mol% of the total content of the double alkali metal oxide MgO-MO, and the molar ratio of K and Na is 1:1. Stir and evaporate to paste at 100°C, dry, extrude, calcined at 700°C for 12 hours, crush to 60 mes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com