Indolocarbazole alkaloid with cytotoxic activity as well as preparation method and application
A technology of indolocarbazole and alkaloids, which is applied in the fields of biotechnology and active compound preparation, and can solve the structure, activity and preparation method of indolocarbazole alkaloids that have not been studied and reported cytotoxic activity, and no indolocarbazole has been reported. problems such as the test results of the toxicity of azoles, to achieve the effects of easy operation and implementation, reduced toxicity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Construction of M1146 / pWLI628 bacterial strain
[0057] The inventor obtained the cosmid pWLI625 containing the spc gene cluster in the early stage, and used the method of homologous recombination to replace the spcMA gene with the apramycin sulfate resistance gene aac(3)IV, and the aac(3)IV gene was digested by XbaI remove. The spcMB gene was then replaced by the aac(3)IV gene, and the aac(3)IV gene was removed by speI enzyme digestion. Load the obtained cosmid with the heterologous expression element aac(3)IV-oriT- -attP / int, introduced into Streptomyces coelicolor M1146 for heterologous expression, and obtained recombinant strain M1146 / pWLI628.
Embodiment 2
[0058] Embodiment 2: the preparation of compound 1-9
[0059] 1) Inoculate the recombinant strain M1146 / pWLI628 into a 500mL Erlenmeyer flask containing 200mL TSBY medium (3% tryptone bean soup powder, 10.3% sucrose, 0.05% yeast extract, 0.1% tryptone, sterilized at 121°C for 20min) Cultivate in a medium shaker, the culture condition is 30°C, 220rpm for 2 days, and obtain the seed liquid;
[0060] 2) The seed solution obtained in step 1) was transferred to Actinomycetes No. 2 medium (1% soluble starch, 2% glucose, 1% yeast extract, 0.3% beef extract, 0.4% corn extract, 0.05% magnesium sulfate, 0.05% potassium dihydrogen phosphate, 0.2% calcium carbonate, sterilized at 121° C. for 20 min) and cultivated on a shaker at 30° C., 220 rpm for 5 days to obtain a fermentation broth.
[0061] 3) centrifuge the fermented liquid obtained in step 2), soak the cells in acetone and ultrasonically crush them, evaporate the acetone to dryness, combine with the supernatant, extract twice with...
Embodiment 3
[0062] Embodiment 3: the characterization of compound 1 and compound 2
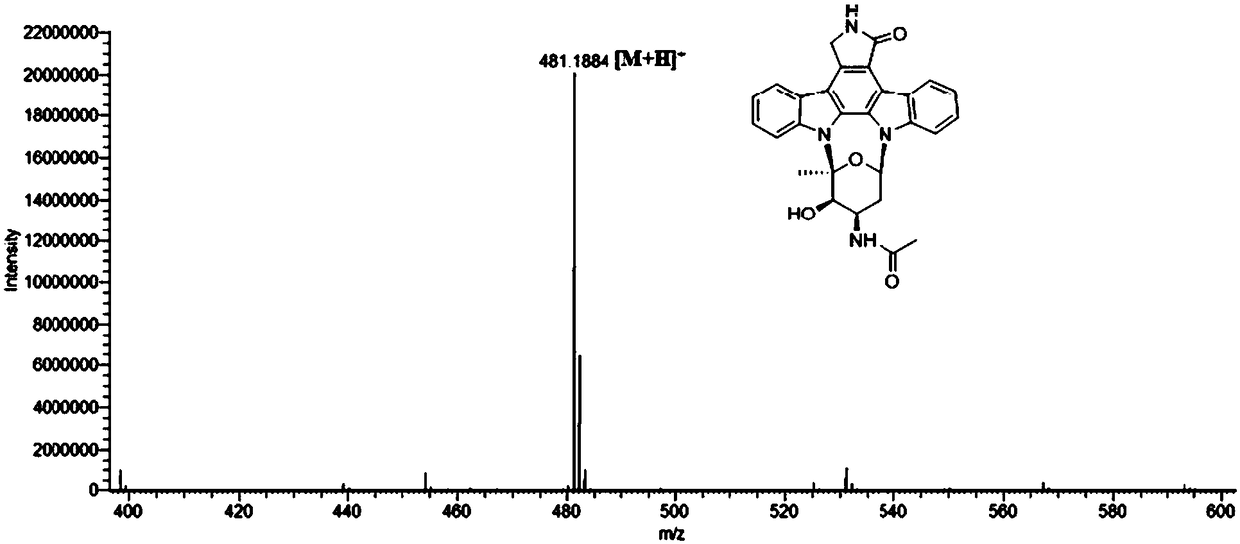
[0063] Compound 1: yellow amorphous powder, molecular formula is C 28 h 24 N 4 o 4 .
[0064] It was identified as 3'-N-acetyl-4'-hydroxylstaurosporine and named as staurosporine M1.
[0065] [α] 21 D +9.8(c 0.05,MeOH); UV(MeOH)λ max (logε)206(4.21),292(4.38),336(3.89),372(3.81)nm; CD(MeOH)λ max (Δε)212(-6.84),247(+1.98),273(-0.75),297.5(+3.93),314.5(+0.28),329(+0.67)nm; HR-ESIMS m / z 481.1864[M+ H] + . See Table 1 for NMR.
[0066] Compound 2: yellow amorphous powder, molecular formula is C 32 h 26 N 4 o 4 .
[0067] It was identified as 3'-[3-methylpyridin-2(1H)-one]-4'-hydroxylstaurosporine and named as staurosporine M3.
[0068] [α] 21 D +198.0(c 0.025,MeOH); UV(MeOH)λ max (logε)244(4.19),292(4.47),336(3.78),372(3.49)nm; CD(MeOH)λ max (Δε)208.5(-10.56),228.5(-2.58),237(-7.48),251.5(+2.21),264(-10.16),298(+39.59),327(+3.84),332.5(+4.05) nm; HR-ESIMS m / z531.2735[M+H] + . See Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com