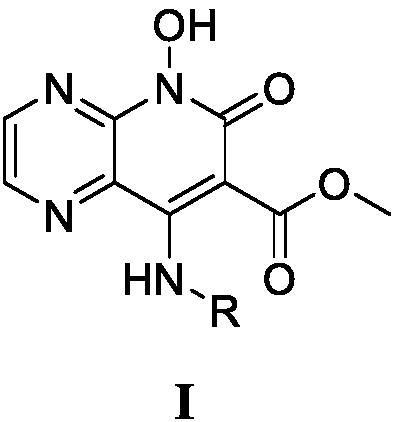
8-amino-7-methyl formate-pyrazine pyridone derivatives and preparation method and use thereof
A technology of pyrazine pyridone and methyl formate, which is used in the preparation of 8-amino-7-methyl formate-pyrazine pyridone derivatives, the application in the field of anti-HIV drugs, the field of derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
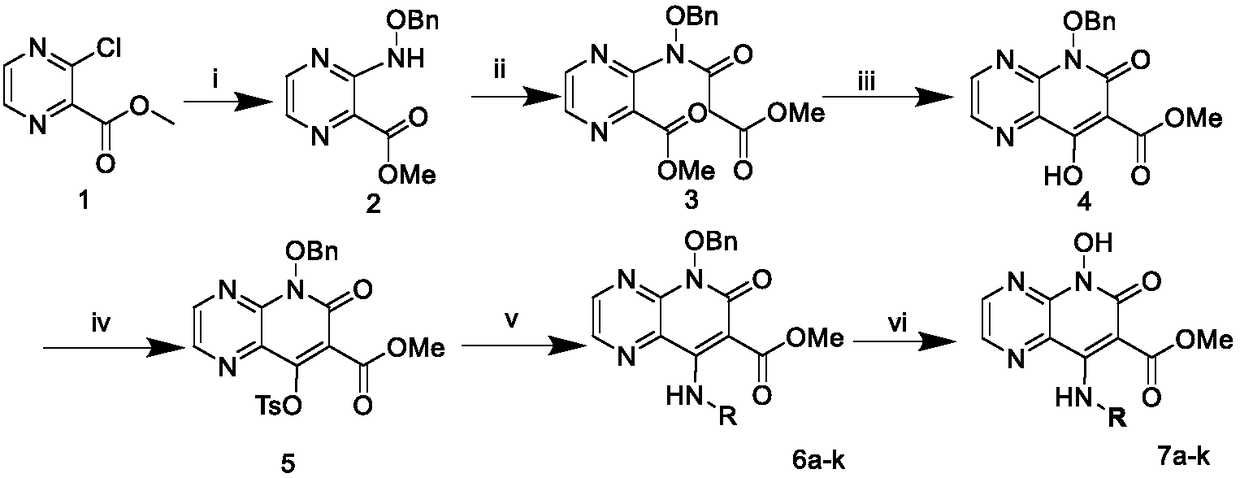
[0048] Embodiment 1: Preparation of intermediate 3-((benzyloxy)amino)pyrazine-2-carboxylate methyl ester (2)
[0049] Methyl 3-chloropyrazine-2-carboxylate (504mg, 2.9mmol, 1eq.), O-benzylhydroxylamine (1073mg, 8.72mmol, 3eq.), dimethylsulfoxide (3mL) and N,N - Diisopropylethylamine (1.44mL, 8.72mmol, 3eq.) was added to a microwave reaction tube, and microwaved at 100°C for one hour. After the reaction is complete, transfer the reaction solution into a separatory funnel, add 30 mL of ethyl acetate and 30 mL of saturated sodium chloride solution, separate the organic phase, extract the water layer twice with ethyl acetate, combine the organic phases, and add anhydrous magnesium sulfate to dry. Filtrate, concentrate the filtrate, mix the sample with 100-200 mesh silica gel, separate and purify by 200-300 mesh silica gel column chromatography (eluent EA:PE=1:4) to obtain the crude intermediate 2, and use petroleum ether / n-hexane for the crude product Recrystallization of alkane ...
Embodiment 2
[0052] Embodiment 2: Preparation of intermediate 3-(N-(benzyloxy)-3-methoxy-3-oxopropionamido)pyrazine-2-carboxylic acid methyl ester (3)
[0053] Intermediate 2 (1897mg, 7.29mmol, 1eq.), triethylamine (2.02mL, 14.59mmol, 2eq.) and 40mL of methylene chloride were added to a 100mL eggplant-shaped bottle, and placed in an ice bath to stir . Under ice bath, malonate monomethyl chloride (3.12 mL, 29.18 mmol, 4 eq.) was slowly added dropwise into the reaction flask. After 30 min, the ice bath was removed, and the reaction was transferred to a 45° C. oil bath under reflux and stirred. After 24 hours, the reaction was completed, the reaction solution was transferred to a separatory funnel, 30 mL of dichloromethane was added, 30 mL of saturated sodium chloride solution was extracted, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, dried by adding anhydrous magnesium sulfate, Filtrate, concentrate the filtrate, mix the sample with 100-200...
Embodiment 3
[0056] Example 3: Intermediate 5-(benzyloxy)-8-hydroxyl-6-oxo-5,6-dihydropyrido[2,3-b]pyrazine-7-carboxylic acid methyl ester (4) preparation
[0057] Intermediate 3 (212mg, 0.557mmol, 1eq.), sodium methoxide (74mg, 1.376mmol, 2.47eq.) and 9.8mL of methanol were added into a 50mL eggplant-shaped bottle, stirred at room temperature overnight. After the reaction is completed, 4N HCl solution is added dropwise to the reaction system to adjust the pH to 3-4. During this process, flocculent white insoluble matter is formed, filtered by suction, and after the filter cake is dried, the intermediate 5-(benzyloxy) -8-Hydroxy-6-oxo-5,6-dihydropyrido[2,3-b]pyrazine-7-carboxylic acid methyl ester (4, yield: 66%), white solid, melting point: 195- 197°C.
[0058] Spectral data:
[0059] 1 H NMR (400MHz, DMSO-d 6)δ8.88 (d, J=2.3Hz, 1H, pyrazine-H), 8.70 (d, J=2.3Hz, 1H, pyrazine-H), 7.64-7.62 (m, 2H, Ph-H), 7.46- 7.39(m,3H,Ph-H),5.17(s,2H,CH 2 ),3.84(s,3H,CH 3 ). 13 C NMR (100MHz, D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com