Water-soluble fluorescent monomer 8-(allyloxy)-1,3,6-pyrene trisulfonic acid and preparation method thereof
A fluorescent monomer, allyloxy technology, applied in the preparation of sulfonic acid, chemical instruments and methods, luminescent materials, etc., can solve problems such as toxicity and environmental pollution, achieve low production cost, good fluorescence performance, and realize online detection. and the effect of automatic drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Add 2.62 g (0.005 mol) of 8-hydroxyl-1,3,6 trisodium pyrenetrisulfonate, 0.80 ml of 50% sodium hydroxide solution (wherein sodium hydroxide is 0.010 mol) in a 100 ml three-necked flask , add 50ml of water, and stir for 15 minutes at room temperature with a heatable magnetic stirrer. After adjusting the temperature to 80°C, add 2.31g (0.030mol) of chloropropene dropwise. The rate of addition of chloropropene is 0.8-1.2g / min. After completion, continue to react at 80°C while stirring for 6 hours, cool to room temperature, and prepare 8-(allyloxy)-1,3,6-pyrenetrisulfonic acid fluorescent monomer was obtained, and the product yield was 91wt%.
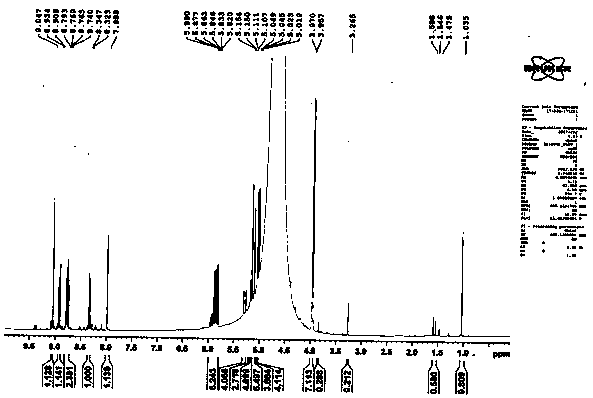
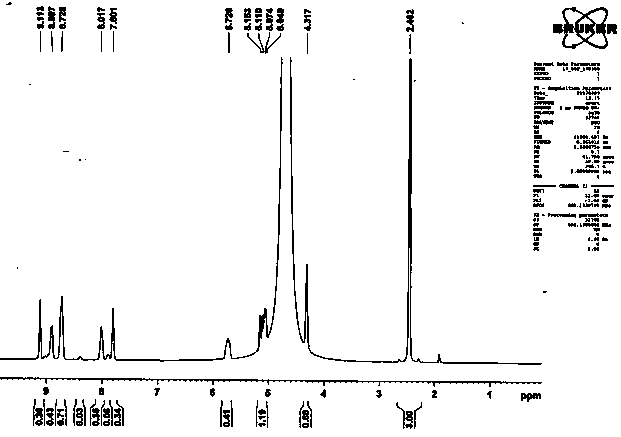
[0021] according to figure 1 and figure 2 The comparison shows that the yield of 8-(allyloxy)-1,3,6-pyrenetrisulfonic acid fluorescent monomer in Example 1 is 91%.
Embodiment 2
[0022] Embodiment 2: Add 2.62g (0.005mol) of 8-hydroxyl-1,3,6 trisodium pyrenetrisulfonate in a 100ml three-necked flask, 0.80ml of 50% sodium hydroxide solution (wherein sodium hydroxide is 0.010mol) , add 50ml of water, and stir for 15 minutes at room temperature with a heatable magnetic stirrer. After adjusting the temperature to 80°C, add 1.54g (0.020mol) of chloropropene dropwise. The rate of addition of chloropropene is 0.8-1.2g / min. After completion, continue to react at 80°C while stirring for 6 hours, cool to room temperature, and prepare 8-(allyloxy)-1,3,6-pyrenetrisulfonic acid fluorescent monomer was obtained, and the product yield was 87wt%.
Embodiment 3
[0023] Embodiment 3: add 2.62g (0.005mol) of 8-hydroxyl-1,3,6 trisodium pyrenetrisulfonate in a 100ml there-necked flask, 0.72ml of 50% sodium hydroxide solution (wherein sodium hydroxide is 0.009mol) , add 50ml of water, and stir for 15 minutes at room temperature with a heatable magnetic stirrer. After adjusting the temperature to 60°C, add 0.693g (0.009mol) of chloropropene dropwise, and the dropping rate of chloropropene is 0.8-1.2g / min. After completion, continue to react at 60°C while stirring for 6 hours, cool to room temperature, and prepare 8-(allyloxy)-1,3,6-pyrene trisulfonic acid fluorescent monomer was obtained, and the product yield was 49 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com