Preparation process for synthesizing 2,6-dihydroxynaphthalene
A technology of dihydroxynaphthalene and preparation process, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc. The problems such as low yield of hydroxynaphthalene and high cost of waste water treatment can achieve the effects of easy obtaining of materials, easy alkali melting, and reduction of dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] A preparation process for synthesizing 2,6-dihydroxynaphthalene, the concrete steps are as follows:
[0019] (1) In the autoclave, add 33-34 parts of sodium 2,6-naphthalene disulfonate, 12-18 parts of sodium hydroxide, and 50-52 parts of water, stir and heat up to 280°C-320°C and at this temperature Stir the reaction for 8-10 hours;
[0020] (2) Stir and cool the solution obtained after the reaction in step (1) to room temperature, add sulfuric acid with a concentration of 40%-60%, and neutralize the reaction until PH=0.5-2;
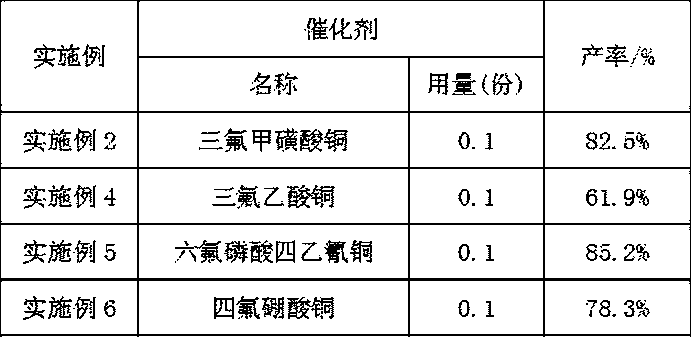
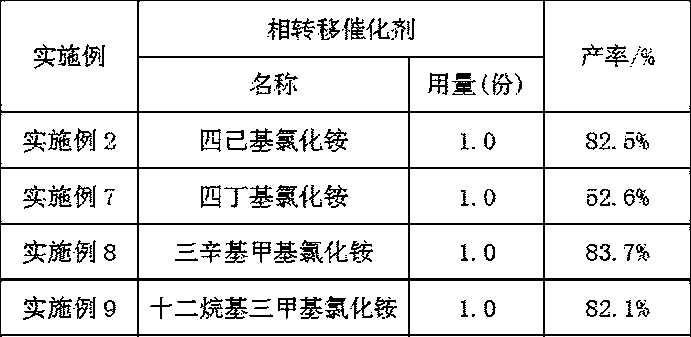
[0021] (3) Filter the suspension obtained in step (2), transfer the filtered solid to a container, add 150-152 parts of hexane, 0.1-0.15 parts of copper salt catalyst, and 1-1.5 parts of phase transfer catalyst, and stir to raise the temperature To 30°C-60°C, add 12-50 parts of an oxidizing agent with a concentration of 20%-30%, and continue to react at a temperature of 30°C-70°C for 2-4 hours to obtain a 2,6-dihydroxynaphthalene solution.
[00...
Embodiment 1
[0028] A preparation process for synthesizing 2,6-dihydroxynaphthalene, the concrete steps are as follows:
[0029] (1) In an autoclave, add 33 parts of sodium 2,6-naphthalene disulfonate, 12 parts of sodium hydroxide, and 50 parts of water, stir and raise the temperature to 280°C and react at this temperature for 8 hours;
[0030] (2) Stir and cool the solution obtained after the reaction in step (1) to room temperature, add sulfuric acid with a concentration of 40%, and neutralize the reaction to PH=0.5;
[0031] (3) Filter the suspension obtained in step (2), transfer the filtered solid to a container, add 150 parts of hexane, 0.1 part of copper trifluoromethanesulfonate catalyst, and 1 part of tetrahexylammonium chloride phase transfer catalyst , stir and heat up to 30°C, add 12 parts of oxidant with a concentration of 20%, continue the reaction at 30°C for 2 hours, stop the reaction, extract the water phase with 50 parts of n-hexane for 3 times, combine the organic phases...
Embodiment 2
[0033] A preparation process for synthesizing 2,6-dihydroxynaphthalene, the concrete steps are as follows:
[0034] (1) In the autoclave, add 33.5 parts of sodium 2,6-naphthalene disulfonate, 16 parts of sodium hydroxide, and 51 parts of water, stir and raise the temperature to 300°C and react at this temperature for 9 hours;
[0035] (2) Stir and cool the solution obtained after the reaction in step (1) to room temperature, add sulfuric acid with a concentration of 50%, and neutralize the reaction until PH=1;
[0036] (3) Filter the suspension obtained in step (2), transfer the filtered solid to a container, add 151 parts of hexane, 0.1 part of copper trifluoromethanesulfonate catalyst, and 1.0 part of tetrahexylammonium chloride phase transfer catalyst , stir and heat up to 50°C, add 40 parts of oxidant with a concentration of 30%, continue the reaction at 50°C for 3 hours, stop the reaction, extract the water phase with 50 parts of n-hexane for 3 times, combine the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com