Method for preparing medical grade valine through fermentation
A valine and pharmaceutical-grade technology, which is applied in the field of fermentation preparation and extraction of pharmaceutical-grade valine, can solve the problems of difficulty in realizing industrial scale and preparation of valine with pharmaceutical-grade purity, and achieves the extension of the acid production cycle and the promotion of Utilization and transport, the effect of less residual sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A method for preparing and extracting pharmaceutical grade valine by fermentation, comprising the steps of:
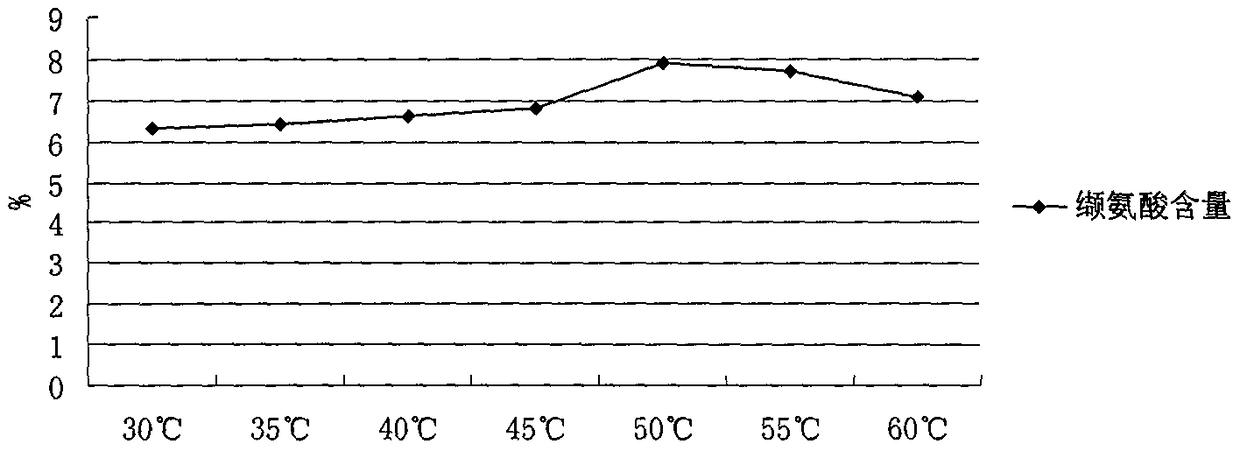
[0039] Brevibacterium lactofermentum ATCC1470 seed liquid (1 × 10 8 cfu / ml) was inserted into the fermentation medium according to the inoculation amount of 7%, and fermented continuously for 55 hours to obtain a valine fermentation liquid; the temperature during the fermentation process was controlled at 30°C, the pH was controlled at 6.5, and the glucose concentration was controlled not less than 20g / L;
[0040] The components of the fermentation medium are: glucose 50g / L, corn steep liquor 30g / L, soybean meal 10g / L, lactose 10g / L, ammonium sulfate 6g / L, potassium dihydrogen phosphate 0.1g / L, magnesium sulfate 0.1g / L, manganese sulfate 6mg / L, ferrous sulfate 6mg / L, potassium chloride 6mg / L;
[0041] Utilize the ceramic membrane to filter the valine fermented liquid to obtain filtrate A (valine content is 4.9%) and wet thalline; Add 3wt% tourmaline powder to...
Embodiment 2
[0049] A method for preparing and extracting pharmaceutical grade valine by fermentation, comprising the steps of:
[0050] Brevibacterium lactofermentum ATCC1470 seed liquid (1 × 10 8 cfu / ml) was inserted into the fermentation medium according to the inoculation amount of 8%, and fermented continuously for 50 hours to obtain valine fermentation broth; the temperature during the fermentation process was controlled at 30°C, the pH was controlled at 6.5, and the glucose concentration was controlled not less than 15g / L;
[0051] The components of the fermentation medium are: glucose 50g / L, corn steep liquor 30g / L, soybean meal 10g / L, lactose 10g / L, ammonium sulfate 6g / L, potassium dihydrogen phosphate 0.1g / L, magnesium sulfate 0.1g / L, manganese sulfate 6mg / L, ferrous sulfate 6mg / L, potassium chloride 6mg / L;
[0052] Utilize ceramic membrane to filter the valine fermented liquid to obtain filtrate A (valine content is 4.7%) and wet thalline; Add 3wt% tourmaline powder to wet th...
Embodiment 3
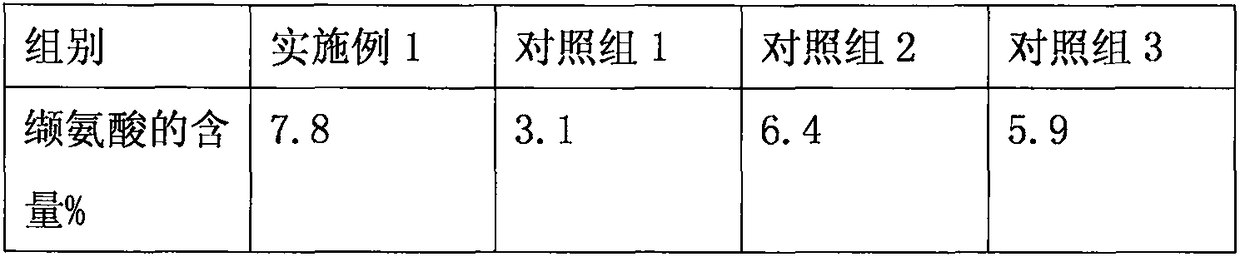
[0060] 1. Taking Example 1 as an example, the content of valine was detected, and the content of valine in the filtrate B was detected respectively; a control group was also set at the same time, wherein, the control group 1 was: only dialysis culture treatment was carried out, and no electric Stone powder and heat treatment, the rest are the same as in Example 1; Control group 2: After the fermentation is completed, tourmaline powder and dialysis culture treatment are carried out, without heat treatment, the rest are the same as in Example 1; Control group 3: After the fermentation is completed, heat treatment and dialysis culture treatment are carried out , do not adopt tourmaline powder to process, and all the other are with embodiment 1; Detect the content of valine in each group filtrate B, and concrete results are shown in Table 1:
[0061] Table 1
[0062]
[0063] Conclusion: As shown in Table 1, through the recultivation treatment of discarded bacterial strains, a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com