Chimeric antigen receptor containing truncated CD20 molecules and lentiviral vector as well as application
A technology of chimeric antigen receptor and lentivirus, which is applied in the field of chimeric antigen receptor and tumor immunotherapy, can solve the problems of side effects, cells and toxicity, and achieve the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of Lv-anti-HER-2CAR lentiviral expression vector
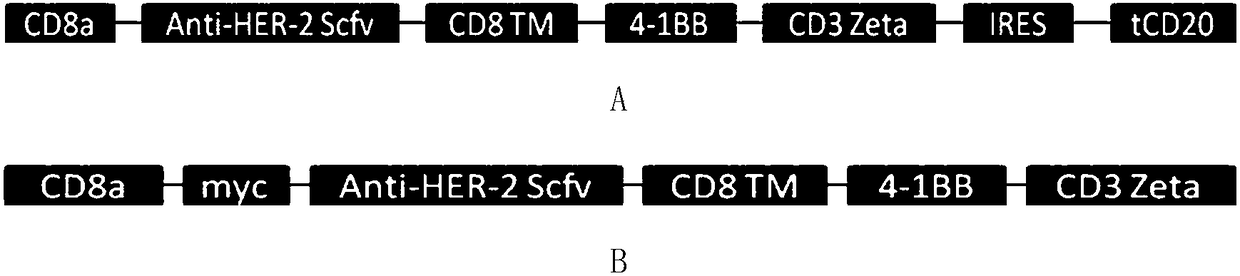
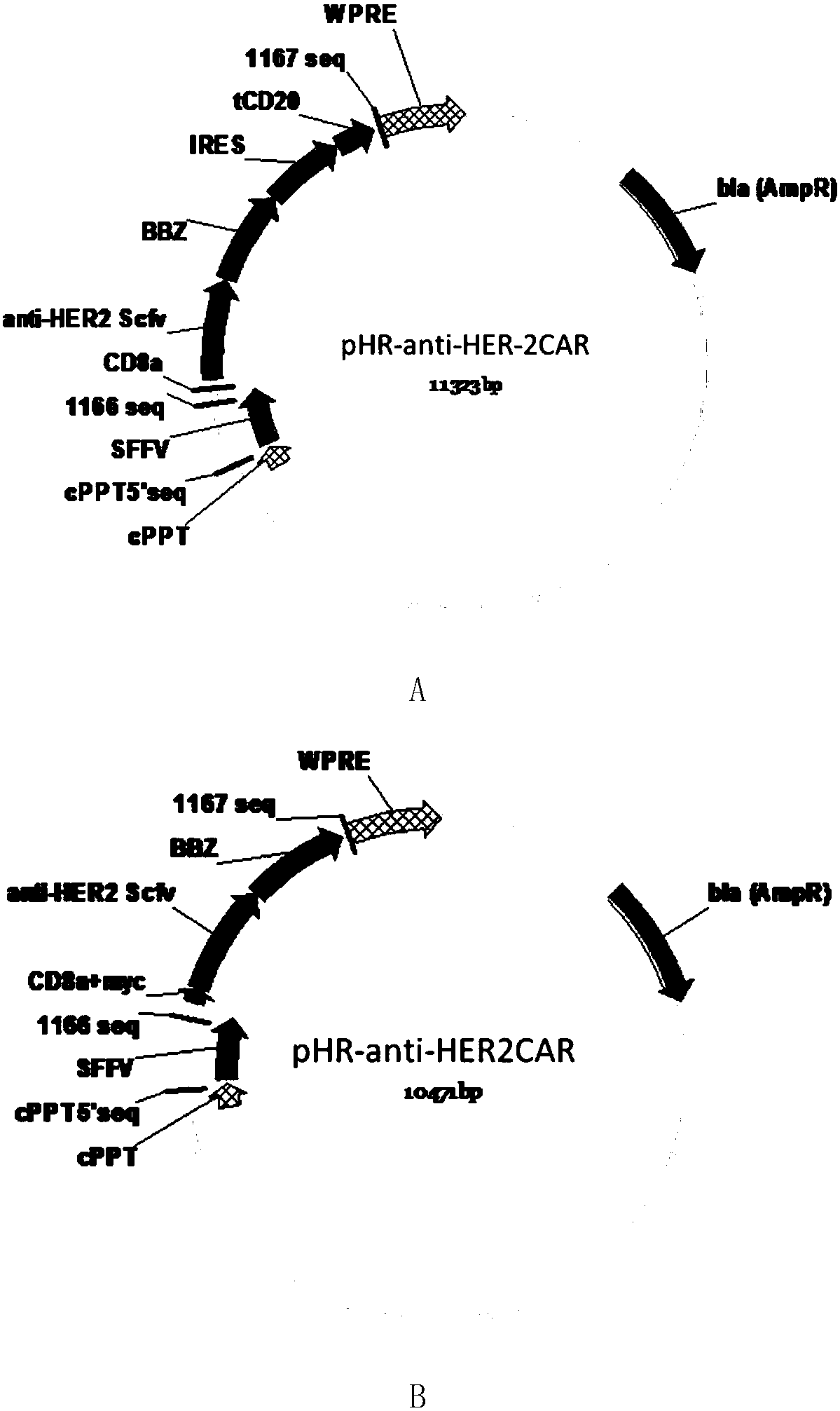
[0065] Such as figure 1 As shown in A, the CD8 transmembrane signal peptide, anti-HER-2Scfv, CD8 transmembrane region, 4-1BB co-stimulatory signal region, and CD3Zeta TCR activation region were sequentially cloned into the lentiviral backbone plasmid pHR to obtain the following figure 2 The pHR-antiHER-2CAR plasmid containing the tCD2 tag shown in A (the nucleotide sequence of the plasmid is shown in SEQ ID NO.1, which expresses a chimeric antigen receptor comprising a truncated CD20 molecule, and its amino acid sequence is shown in SEQ ID NO.1 shown in ID NO.2). Such as figure 1 As shown in B, the CD8 transmembrane signal peptide, myc tag, anti-HER-2Scfv, CD8 transmembrane region, 4-1BB co-stimulatory signal region, and CD3Zeta TCR activation region were sequentially cloned into the lentiviral backbone plasmid pHR to obtain the following: figure 2 The pHR-antiHER-2 CAR plasmid containing myc ...
Embodiment 2
[0067] Example 2 Determination of virus titer
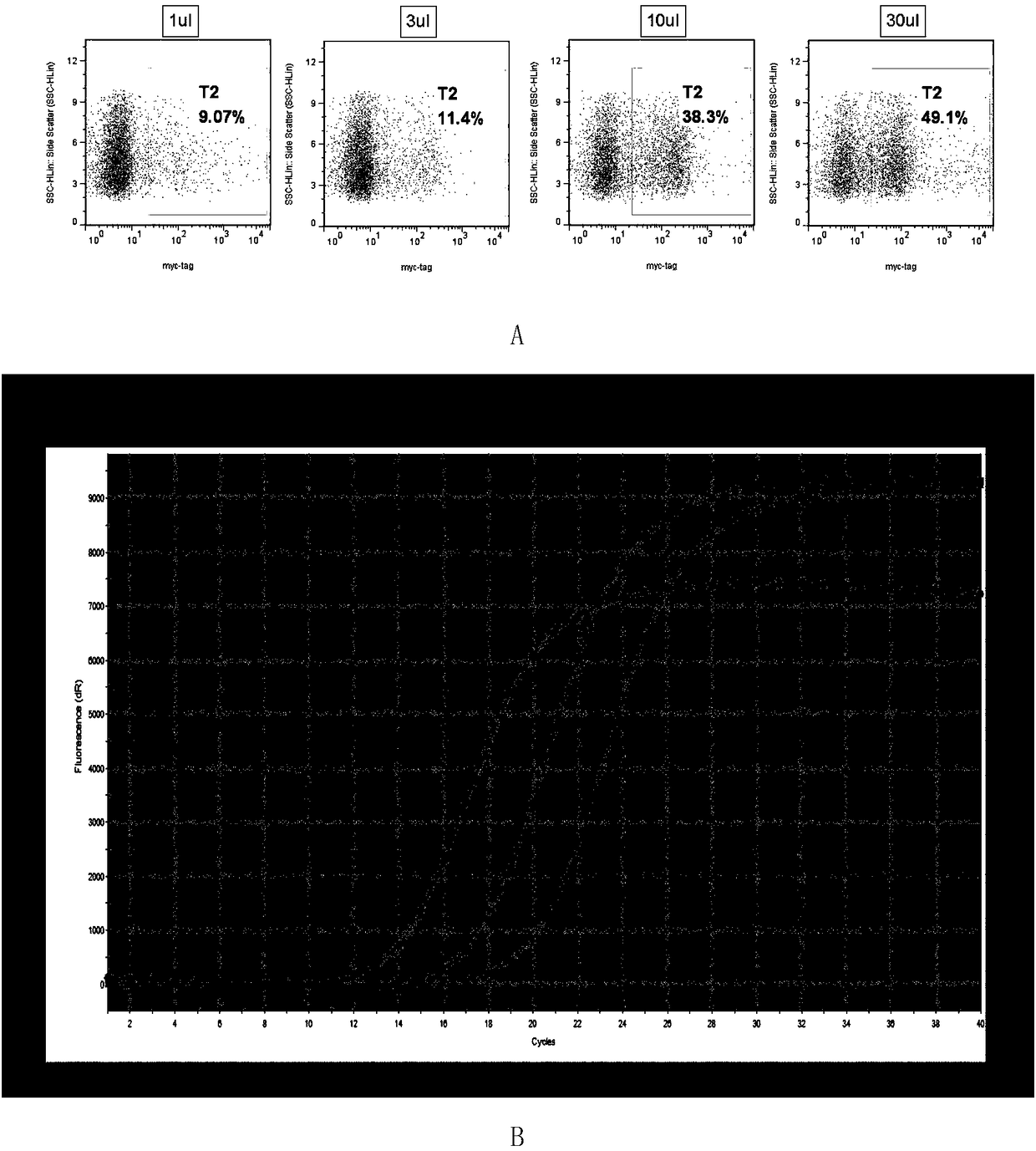
[0068] A cell infection method: 1 × 10 per well in 24 wells 5 For each K562 cell, take the concentrated virus and dilute it 10 times, add 1 μL, 3 μL, 10 μL, 30 μL virus respectively, and store at 37°C in 5% CO 2 After culturing for 48 hours, 200 μL of cell liquid was taken from each well for flow cytometric detection. Add 1 μL myc-tag primary antibody (CST, cat: 2276) to each sample, incubate at room temperature in the dark for 15 minutes, add 2 μL PEanti-mouse Ig light chain lambda (Biolegend, cat: 407308) secondary antibody to each sample, and incubate at room temperature in the dark for 15 minutes , centrifuged at 400 g for 5 min, and the pellet was resuspended in 200 μL of PBS and tested on a machine (Millipore guava easyCyte HT). The result is as image 3 As shown in A, as the amount of virus added increases, the proportion of positive cells gradually increases. When the positive rate is less than or equal to 10%, the nu...
Embodiment 3
[0070] Example 3 Preparation of CAR-T cells
[0071] Take 50 mL of fresh blood, and conduct density gradient centrifugation with lymphocyte separation medium (Tianjin Haoyang) to separate mononuclear cells. Divide mononuclear cells into 1-2 x 10 6 / mL resuspended into X-VIVO 15 medium (Lonza), and at the same time add CD3 monoclonal antibody (Ebioscience, cat: 160037) 50ng / mL and CD28 monoclonal antibody (Novoprotein, cat: GMP-A063) 50ng / mL to activate T Lymphocytes, 37°C 5% CO 2 Incubate for 48 hours.
[0072] Take 2×10 6 Dilute the aliquoted virus with the same medium according to MOI=5, add IL-2 (Quangang) and 4ug / mL polybrene (Sigma) at a final concentration of 500U / mL at the same time, mix well, and store at 37°C in 5% CO 2 Cultivate for 6-8 hours, centrifuge at 300g for 5min to change the medium into fresh X-VIVO 15 medium (containing 500U / mL IL-2).
[0073] Add fresh X-VIVO 15 medium (containing 500U / mL IL-2) every 2-3 days to maintain the cell density at 1×10 6 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com