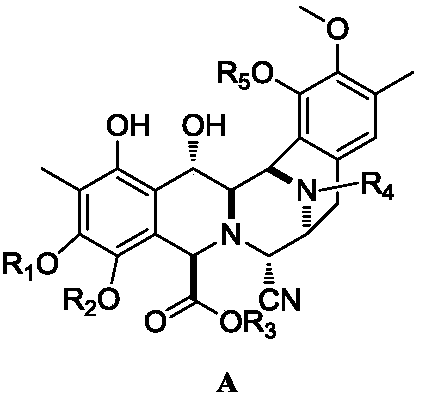
Intermediate product of trabectedin as well as preparation method and application thereof
A technology of racemates and tautomers, which is applied in the key intermediate of trabectedin and its preparation field for the treatment of advanced soft tissue sarcoma, can solve the problem that the influence of reaction temperature is very large, the reaction time is too long, and the reaction system changes. Miscellaneous problems, to achieve the effect of shortening the number of reaction steps, controllable reaction and stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
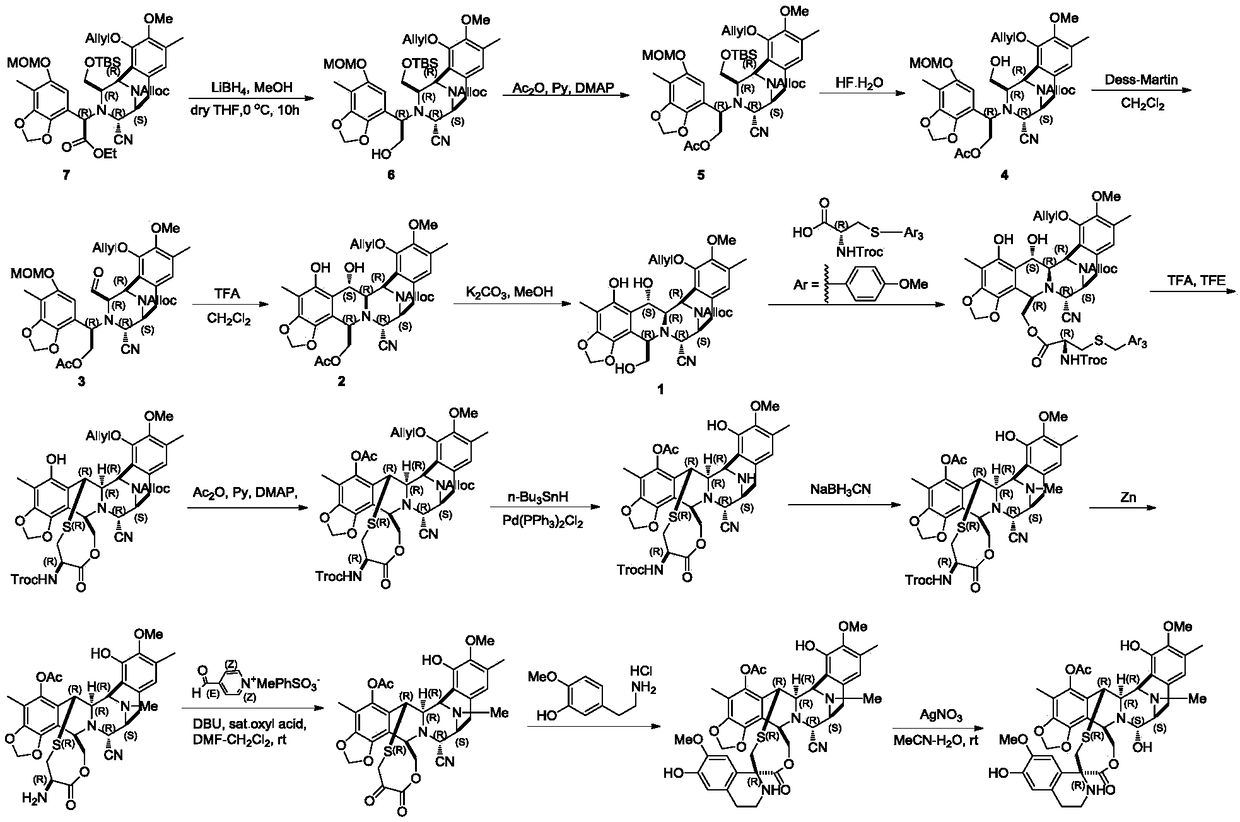
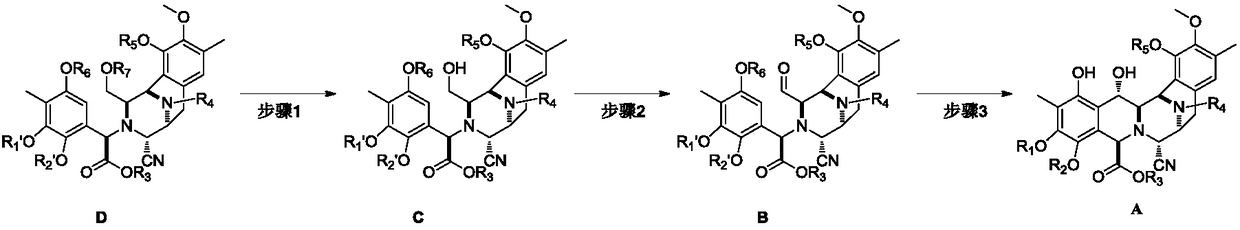
[0042] Embodiment 1: the preparation of compound C-1
[0043]
[0044] Dissolve compound D-1 (34g, 42.08mmol) in acetonitrile (500mL) at room temperature, add pyridine hydrogen fluoride (62.55g, 0.631mol), stir at room temperature until the reaction of raw materials monitored by TLC is complete, and the reaction solution is washed with dichloromethane (1.5L) Diluted, washed successively with saturated sodium bicarbonate solution (500mL), saturated brine (500mL), dried over anhydrous sodium sulfate, filtered, concentrated, crude product flash column chromatography (ethyl acetate:petroleum ether=1:4), Concentration gave colorless oily compound C-1 (28g, yield: 96%, 1 H-NMR (400MHz, CDCl 3 ): δ6.59-6.58(s,1H),6.20(s,1H),6.18-6.10(m,1H),5.88-5.81(m,1H),5.80(s,1H),5.66(s,1H ),5.60-4.99(m,6H),4.81-4.52(m,7H),4.23(m,3H),3.88-3.80(m,2H),3.50-3.48(m,2H),3.49(s,3H ),3.45(s,3H),3.41-3.28(m,1H),3.08-2.95(m,1H),2.22(s,3H),2.11(s,3H),1.30-1.27(m,3H)) .
Embodiment 2
[0045] Embodiment 2: the preparation of compound B-1
[0046]
[0047] Compound C-1 (28g, 40.36mmol) was dissolved in dichloromethane (600mL) at room temperature, and Dess-Martin oxidant (51.35g, 121.08mmol) was added, stirred at room temperature for 0.5 hours, and the reaction of raw materials was monitored by TLC. The reaction solution was diluted with ether (600mL), filtered, and the filtrate was washed successively with saturated sodium bicarbonate solution (150mL) and saturated brine (150mL), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a colorless oily compound B-1 ( 26.52g, yield: 95%, 1 H-NMR (400MHz, CDCl 3 ): δ9.03(d,0.5H),8.84(d,0.5H),6.69-6.66(s,1H),6.23(d,1H),6.08-5.90(m,1H),5.88(s,1H ),6.01-5.82(m,1H),5.78(s,1H),5.75-5.56(d,1H),5.45-4.50(m,10H),4.46-4.00(m,6H),3.71-3.73(s ,3H),3.42-3.40(s,3H),3.18-3.06(m,1H),2.62-2.52(m,1H),2.22(s,3H),2.02(s,3H),1.21-1.18(m ,3H)).
Embodiment 3
[0048] Embodiment 3: the preparation of compound A-1
[0049]
[0050] Compound B-1 (26g, 37.58mmol) was added to a three-necked flask at room temperature, argon was replaced, and trifluoroacetic acid / dichloromethane (1.7L, v / v=1 / 200, wherein trifluoroacetic acid was 112.74 mmol), stirred at room temperature until the TLC monitoring reaction was complete; the reaction solution was diluted with dichloromethane (3L), and washed successively with saturated sodium bicarbonate solution (1L), saturated sodium chloride (1L), dried over anhydrous sodium sulfate, Suction filtration, concentration, column chromatography (EA / PE=1 / 5) separation, concentration to obtain off-white solid compound A-1 (23.12g, yield: 95%, 1 H-NMR (400MHz, CDCl 3 ): δ9.63-9.57(d,1H),6.76(s,1H),6.30-6.08(m,1H),6.10-5.10(m,9H)4.89-3.86(m,9H),3.82(s, 3H),3.69-3.60(m,1H),3.28-3.12(m,2H),2.77(dd,J=17.7,8.1Hz,1H),2.08(s,3H),1.52(s,3H),1.24 (t,3H)).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com