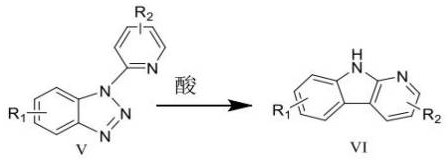
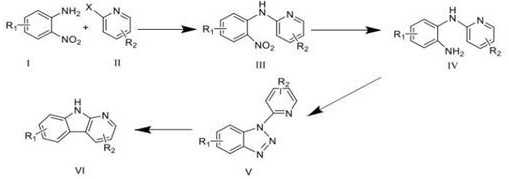
A kind of synthetic method of carboline compounds
一种合成方法、化合物的技术,应用在卡波林类化合物的合成领域,能够解决反应条件苛刻、不易操作、缺乏灵活性等问题,达到降低反应温度、反应条件温和、缩短反应时间的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Taking the preparation of 2-methyl-3-bromo-9H-pyridine[2,3-b]indole as an example
[0073] The synthetic process steps are: (1) Compound 2,5-dibromo-6-picoline (2.70g, 10.76mmol) and o-nitroaniline (1.78g, 12.91mmol) are dissolved in DMF (30mL), and t -BuOK (3.62 g, 32.28 mmol), 5% Pd / C (474.68 mg, 2.96 mmol), vented and protected with nitrogen, the mixture was stirred at 120 °C for 5 h, cooled, dissolved in 20 mL of water, extracted with EtOAc, The extract was washed with water and saturated brine successively, and anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and purify the residue by silica gel column chromatography to obtain the product 5-bromo-o-nitroanilino-6-methylpyridine in a yield of 66%;
[0074] (2) 5-Bromo-o-nitroanilino-6-methylpyridine (2.41 g, 6.8 mmol) was dissolved in EtOAc (20 mL), and SnCl was added 2 (6.46 g, 34.08 mmol), the mixture was stirred and refluxed at 80 °C for 3 h, cooled, dissolved in 20 mL of water, and diss...
Embodiment 1-1
[0078] In the aforementioned step (1), the mass ratio of 2,5-dibromo-6 picoline and o-nitroaniline is selected to be 1:1, 2:1, 3:1; the volume mass of solvent DMF and o-nitroaniline The ratios are 15:1, 16:1, 17:1, 18:1; the molar ratio of 5% Pd / C to o-nitroaniline is 0.2:1, 0.3:1, 0.4:1, 0.5:1; t- The molar ratio of BuOK to o-nitroaniline was 2:1, 3:1, 4:1. The product 5-bromo-o-nitroaniline-6-methylpyridine was obtained, and the average yield was 64.8%; but the molar ratio of acid scavenger t-BuOK and o-nitroaniline was selected to be 1:1, and the average yield of the product was 1:1. The yield was 42.5%, which was not ideal.
Embodiment 1-2
[0080] In the aforementioned step (2), the volume (mL) molar ratio of EtOAc and 5-bromo-o-nitroanilino-6-picoline was selected as 20:1, 30:1, 40:1; iron powder-chlorinated The molar ratio of amine (1:3) to 5-bromo-o-nitroanilino-6-methylpyridine was 4.5:1, 5:1, 5.5:1, 6:1. The average yield of the product 5-bromo-o-aminoanilino-6-methylpyridine is over 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com