Application of progestational hormone in preparing glycogen synthase kinase-3beta inhibitor medicine, and glycogen synthase kinase-3beta inhibitor
A technology for glycogen synthesis and progesterone, which is applied in the field of biomedicine, can solve problems such as complex composition, difficult popularization, cumbersome preparation methods, etc., and achieve the effect of increasing expression and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Preparation of PR2005
[0031] Dissolve progesterone / 17α-hydroxyprogesterone caproate in 1% DMSO solvent, vibrate for 5 minutes, and freshly prepare small-dose PR2005: 0.03mg / L, medium-dose PR2005: 0.15mg / L, and large-dose PR2005: 0.3mg / L solution.
Embodiment 2
[0032] Embodiment 2. making animal model
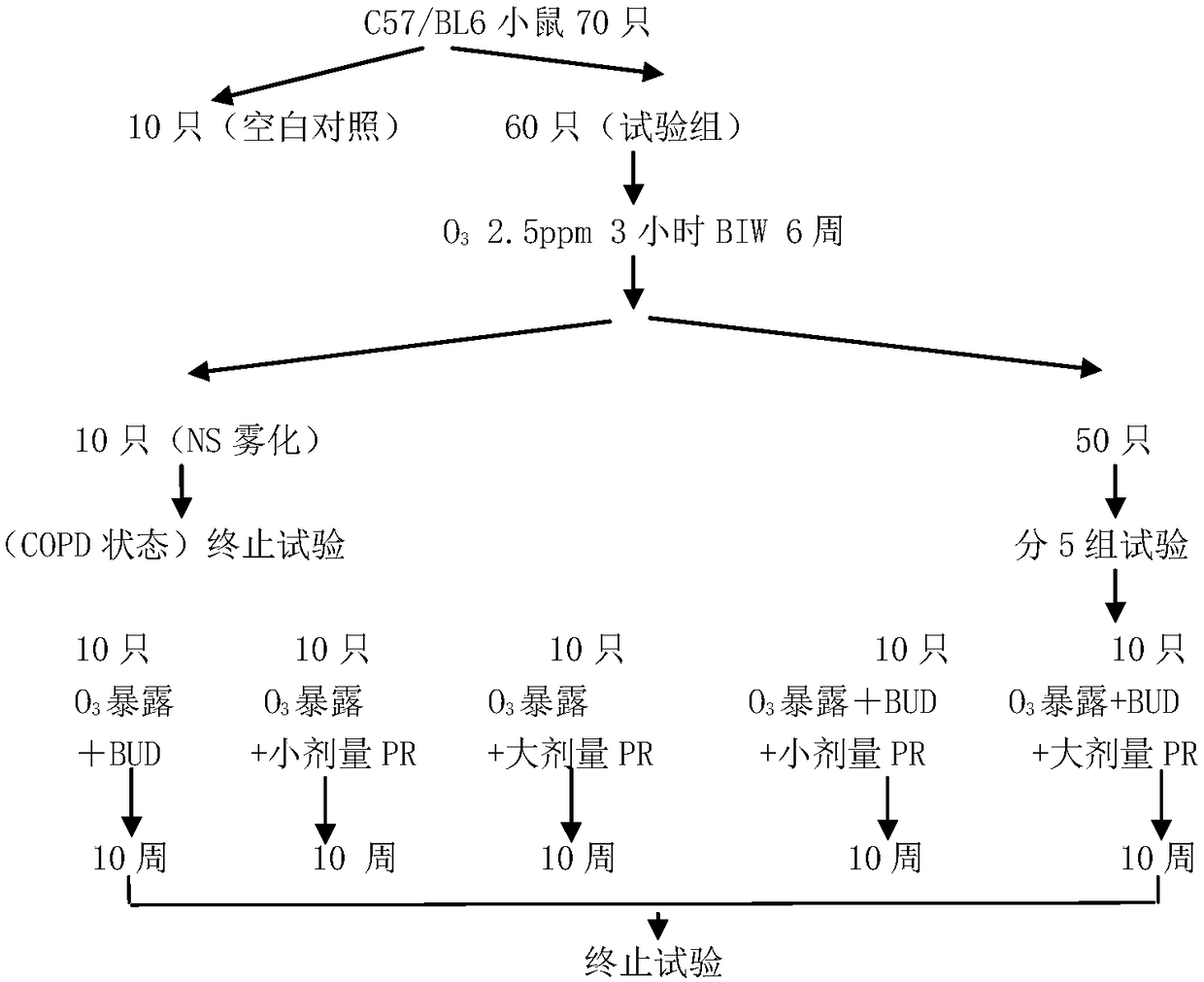
[0033] Seventy healthy and clean male C57 / BL6 mice aged 10 weeks were selected. The animals were acclimated to the environment for one week before the experiment. They were randomly divided into experimental group and control group according to the method shown in the figure below, and the chronic COPD model was established in the experimental group according to the following method.
[0034] The specific method of establishing a chronic COPD model: All mice inhale 2.5ppm O3 in a special O3 inhalation container for 3 hours, twice a week, for 6 weeks. After 6 weeks, continue to inhale O3 according to this method, and the experimental group is randomly divided into Normal saline (NS) nebulization group, Budesonide (BUD) nebulization group, low-dose PR2005 treatment group, high-dose PR2005 treatment group, desonide + low-dose PR2005 treatment group, desonide + medium-dose PR2005 treatment group group, budesonide + high-dose PR2005 treat...
Embodiment 3
[0037] Example 3. Examination of bronchoalveolar lavage fluid
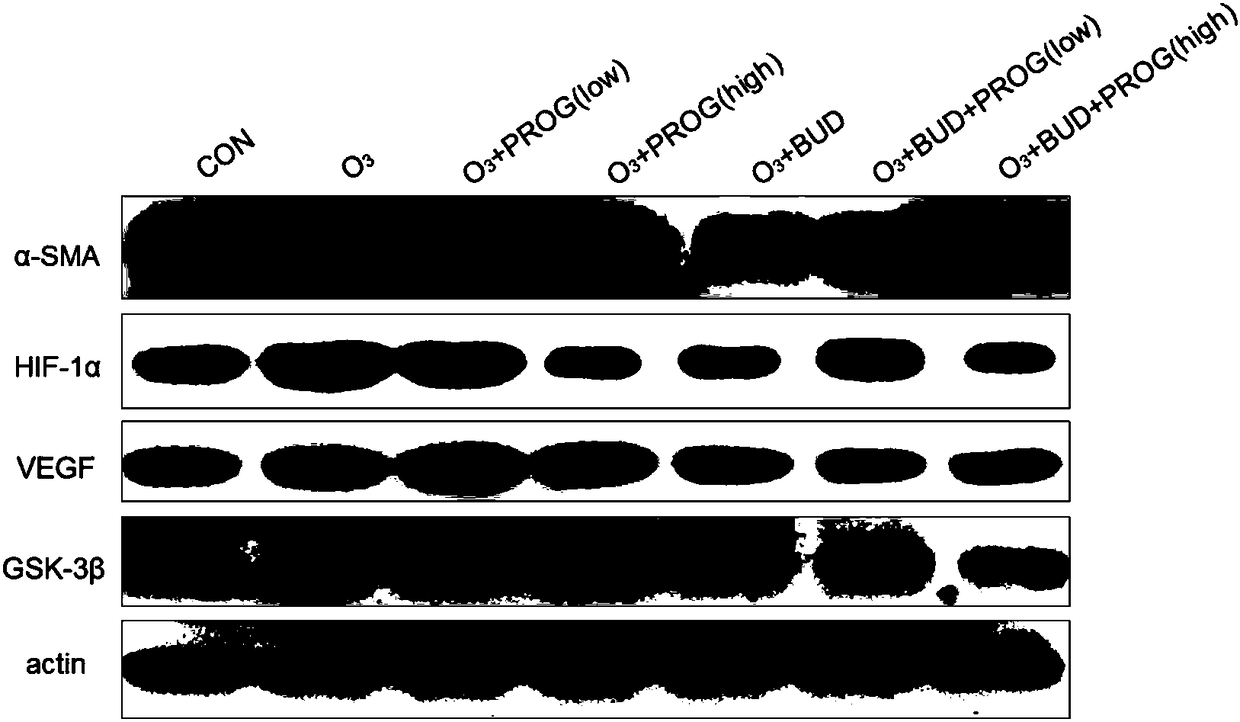
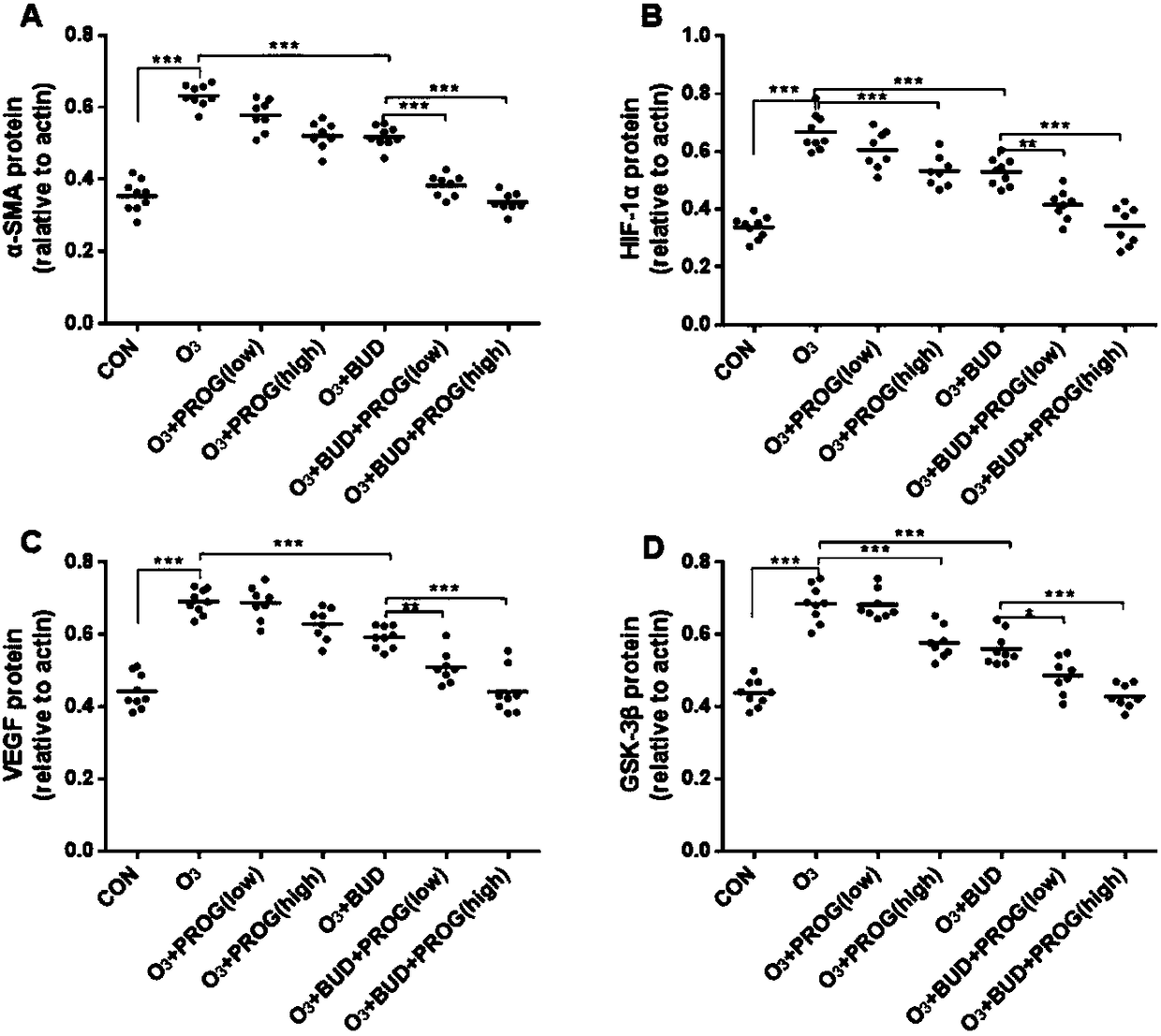
[0038] Use 0.4ml, 0.4ml, 0.4ml of sterile saline at 4°C to lavage the endotracheal tube three times, and the recovery rate is greater than 80% as a qualified specimen. After centrifugation at 1500r / min for 10min, the supernatant was taken out and stored at -80°C. The supernatant was detected by ELISA method for matrix metalloproteinase-8 (MMP-8), MMP-9 and other cytokines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com