Catalyst for efficiently removing formaldehyde in air or waste water at room temperature as well as preparation method thereof
A catalyst and high-efficiency technology, applied in the field of catalysis, can solve the problem of high consumption of precious metals, and achieve the effects of reducing production costs, excellent formaldehyde removal performance, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Catalyst preparation
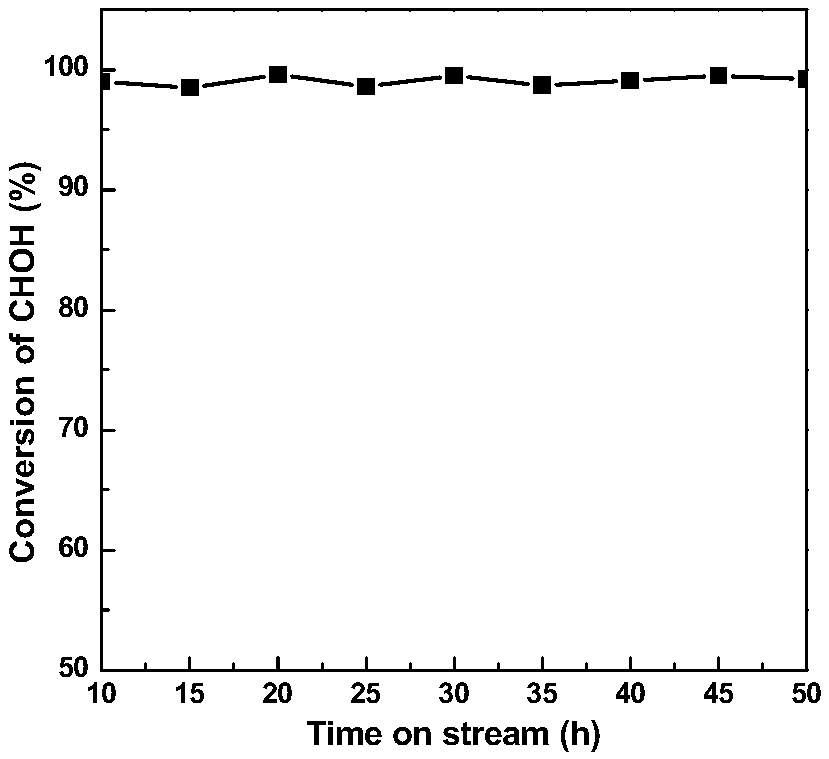
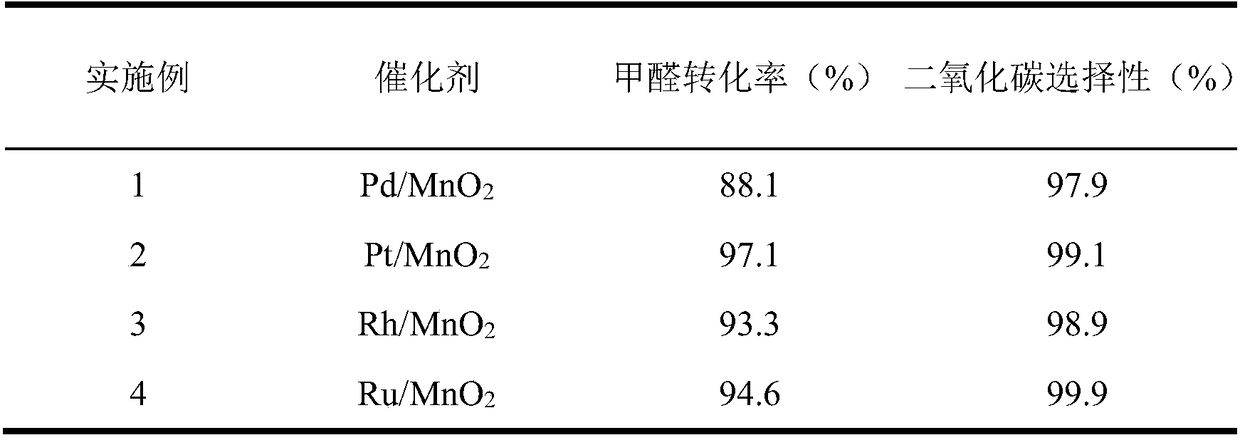
[0033] Weigh 2.74g Mn(NO 3 ) 2 4H 2 O was dissolved in 200ml deionized water and stirred for 30min to obtain solution A. Weigh 1.15g KMnO 4 Dissolve in 100ml deionized water and stir for 30min to prepare solution B. Solution B was slowly added dropwise to solution A, and 0.2 mol / L KOH solution was added dropwise to adjust the pH of the reaction mixture to 10. Add 1.26mL 1mg / mL PdCl 2 After solution, the reaction mixture was transferred to a hydrothermal reaction kettle for hydrothermal reaction at 100° C. for 8 h. After cooling to room temperature, filter, wash and dry, N 2 Pd / MnO was prepared by calcination at 400℃ for 4h in atmosphere 2 catalyst.
[0034] Before the formaldehyde catalytic oxidation reaction, the catalyst needs to be pre-reduced with hydrogen at 350°C for 1h, and then react in a continuous flow fixed bed with a reaction space velocity of 15000h -1 , the formaldehyde pollutant concentration is 500ppm, room temperature, n...
Embodiment 2
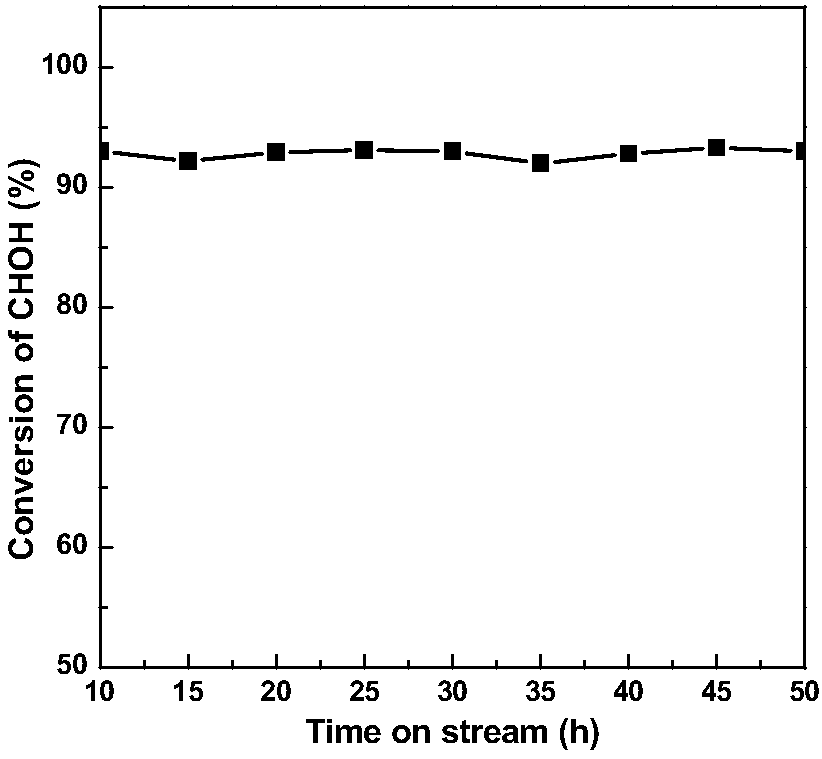
[0036] Catalyst preparation is with reference to embodiment 1, Mn(NO 3 ) 2 4H 2 O is replaced by MnSO 4 , KOH solution was replaced by NaOH solution, PdCl 2 The solution was changed to 1.98mL 1mg / mL H 2 PtCl 6 ·6H 2 O solution, and the rest of the conditions were the same, and Pt / MnO was prepared 2 catalyst. Evaluation conditions are the same as in Example 1, and the evaluation results are shown in Table 1.
Embodiment 3
[0038] Catalyst preparation is with reference to embodiment 1, Mn(NO 3 ) 2 4H 2 O is replaced by Mn(CH 3 COO) 2 4H 2 O, KOH solution is replaced by ammonia water, PdCl 2 The solution was changed to 1.92mL 1mg / mL RhCl 3 ·3H 2 O solution, the rest of the conditions are the same, and Rh / MnO 2 catalyst. Evaluation conditions are the same as in Example 1, and the evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com