Live vaccine diluent as well as preparation method and application thereof and vaccine product
A live vaccine and diluent technology, applied in the field of vaccines, can solve the problems of being susceptible to maternal antibody interference, short antibody maintenance time, and long production time, so as to improve animal antibody content, reduce infection and morbidity, and be easy to operate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
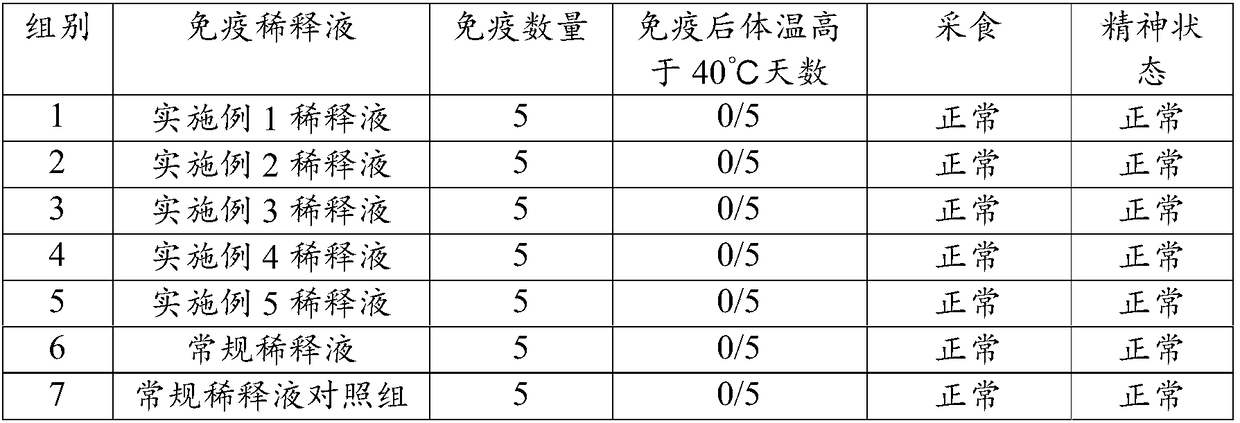
[0033] The present embodiment provides a kind of live vaccine diluent, comprising:
[0034] Carbomer, levamisole and ginsenoside are dissolved in normal saline, and the control final concentration is: carbomer 1% (W / V), levamisole 5% (W / V) and ginsenoside 0.01% (W / V ), using sodium hydroxide or hydrochloric acid to adjust the pH to 7.2 to obtain a live vaccine dilution.
[0035] Dispense according to a certain volume and sterilize under high pressure at 121°C for 15 minutes before use.
Embodiment 2
[0037] The present embodiment provides a kind of live vaccine diluent, comprising:
[0038] Carbomer, levamisole and notoginsenoside are dissolved in water, and the control final concentration is: carbomer 0.02% (W / V), levamisole 1% (W / V) and notoginsenoside 0.001% (W / V ), using sodium hydroxide or hydrochloric acid to adjust the pH to 7.0 to obtain a live vaccine dilution.
[0039] Dispense according to a certain volume and sterilize under high pressure at 121°C for 20 minutes before use.
Embodiment 3
[0041] This embodiment provides a kind of live vaccine diluent, and it contains: 0.01% (W / V) carbomer, 0.1% (W / V) levamisole, 0.0001% (W / V) soybean saponin, 100mM sodium chloride , 0.5mM potassium chloride, 100mM disodium hydrogen phosphate and 0.1mM potassium dihydrogen phosphate.
[0042] The preparation method of this live vaccine diluent is:
[0043] According to the formula, the above substances are dissolved in water for injection, and sodium hydroxide is added to adjust the pH to 7.2. After being dispensed according to a certain volume, it is autoclaved at 121°C for 25 minutes and then used for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com