A method for producing calcium ferrite for polyaluminum ferric chloride by using pickling tank slag
A technology of polyaluminum ferric chloride and calcium ferrite, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of complex production process of polyaluminum ferric chloride, achieve good water control effect and improve quality. The effect of high content and mass percentage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In 5kg iron-containing waste acid solution (wherein Fe 2 o 3 The mass percentage content is 55.2%), first add 1.294kg of heavy calcium carbonate, then add 125g of sodium phosphate, after stirring evenly, sinter in the experimental muffle furnace at 800°C for 2h, cool to room temperature, crush, and sieve to obtain Calcium ferrite, the particle size of calcium ferrite after sieving is 0.08mm;
[0016] In a 1L flat-bottomed flask, add 500mL of 30% hydrochloric acid, 100mL of distilled water, and 125g of aluminum hydroxide, stir and react at 80°C, first heat and boil for 3 hours, then add 100mL of distilled water, then add 82g of calcium ferrite, continue heating and boiling for 1 hour , and suction filtration to obtain the finished polyaluminum ferric chloride.
Embodiment 2
[0018] In 6.666kg iron-containing waste acid solution (wherein Fe 2 o 3 The mass percent content is 55.2%), first add 1.725kg of heavy calcium carbonate, then add 150g of sodium phosphate, after stirring evenly, sinter in an experimental muffle furnace at 600°C for 24h, cool to room temperature, crush, and sieve to obtain Calcium ferrite, the particle size of calcium ferrite after sieving is 0.05mm;
[0019] In a 1L flat-bottomed flask, add 500mL of 30% hydrochloric acid, 100mL of distilled water, and 150g of aluminum hydroxide. Stir the reaction at 100°C and heat and boil for 3 hours, then add 100mL of distilled water, then add 82g of calcium ferrite, and continue heating and boiling for 1 hour Suction filtration to obtain the finished polyaluminum ferric chloride.
Embodiment 3
[0021] In 4.5kg iron-containing waste acid solution (wherein Fe 2 o 3 The mass percent content is 55.2%), first add 1.165kg of heavy calcium carbonate, then add 113g of sodium phosphate, after stirring evenly, sinter in an experimental muffle furnace at 900°C for 1h, cool to room temperature, crush, and sieve to obtain Calcium ferrite, the particle size of calcium ferrite after sieving is 0.01mm;
[0022] In a 1L flat-bottomed flask, add 500mL of 30% hydrochloric acid, 100mL of distilled water, and 113g of aluminum hydroxide, stir and react at 100°C and heat to boil for 3 hours, then add 100mL of distilled water, then add 82g of calcium ferrite, continue heating and boiling for 1 hour, Suction filtration to obtain the finished polyaluminum ferric chloride.
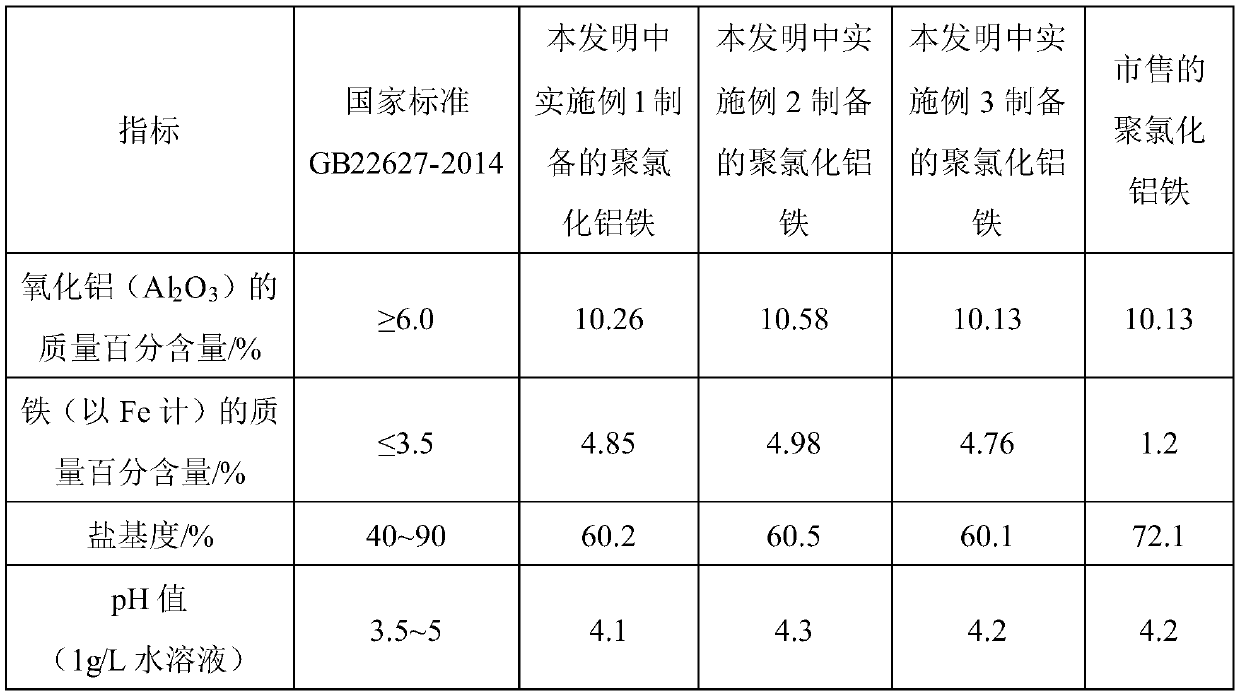
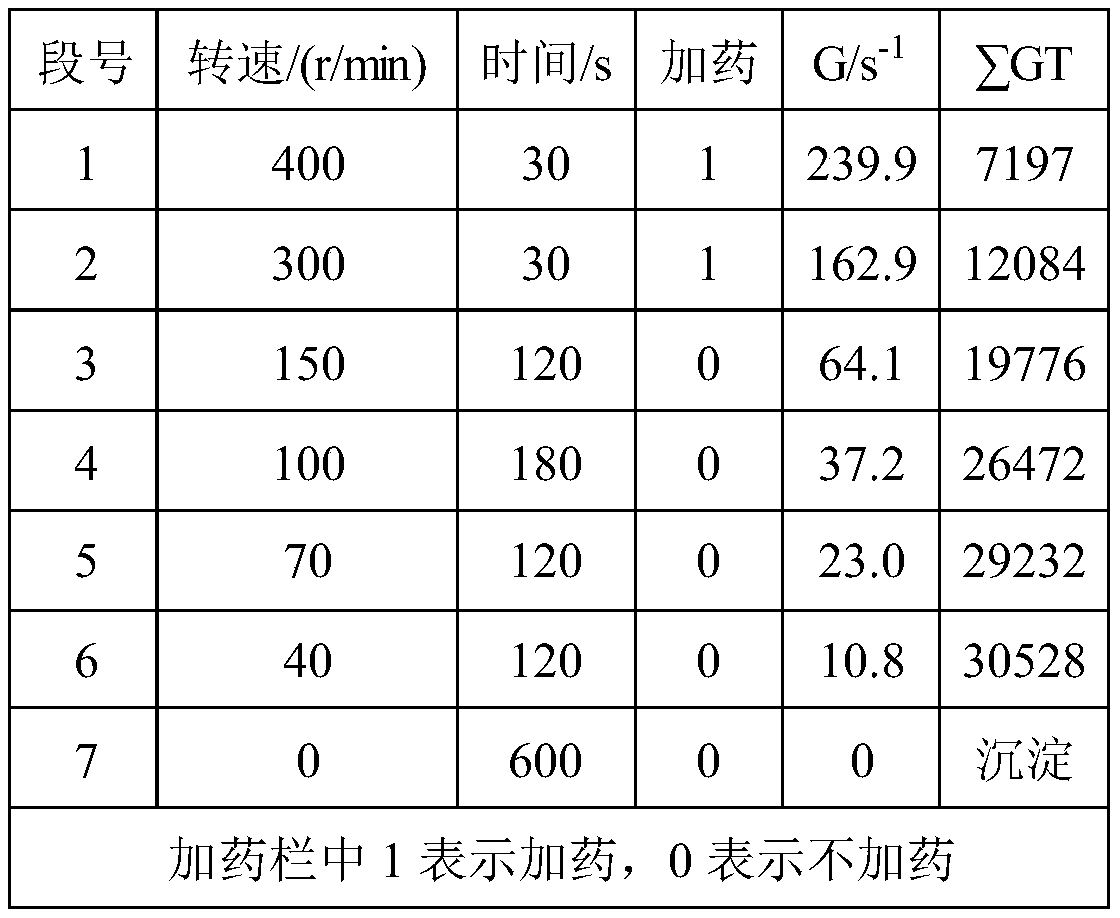
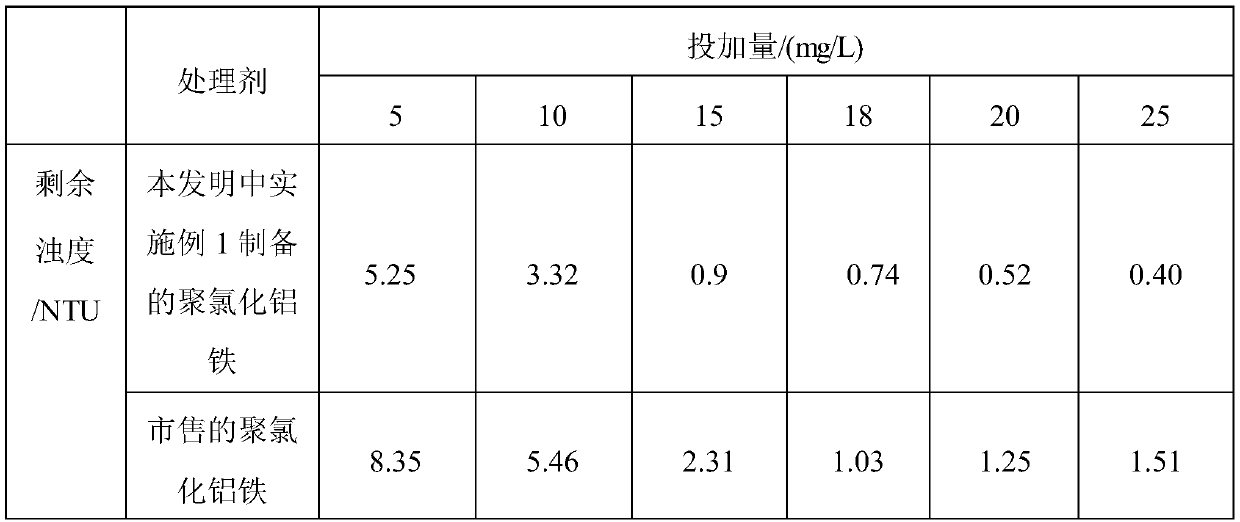
[0023] See Table 1 for the index comparison with commercially available polyaluminum ferric chloride. The following commercially available polyaluminum ferric chloride refers to the polyaluminum ferric chloride produced ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com